Question: Please do 1-3. The 1st image is my data from lab.I think I did 1 and 2 right but I wanted to check if they
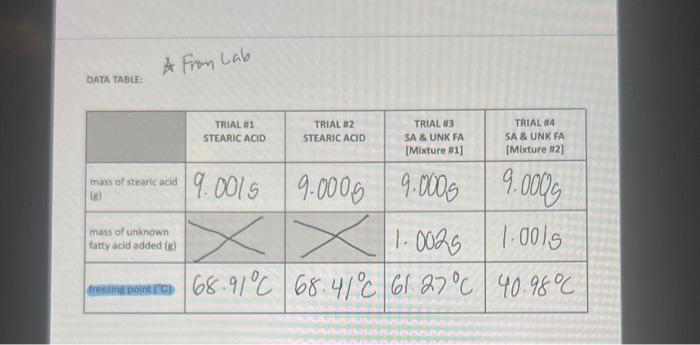



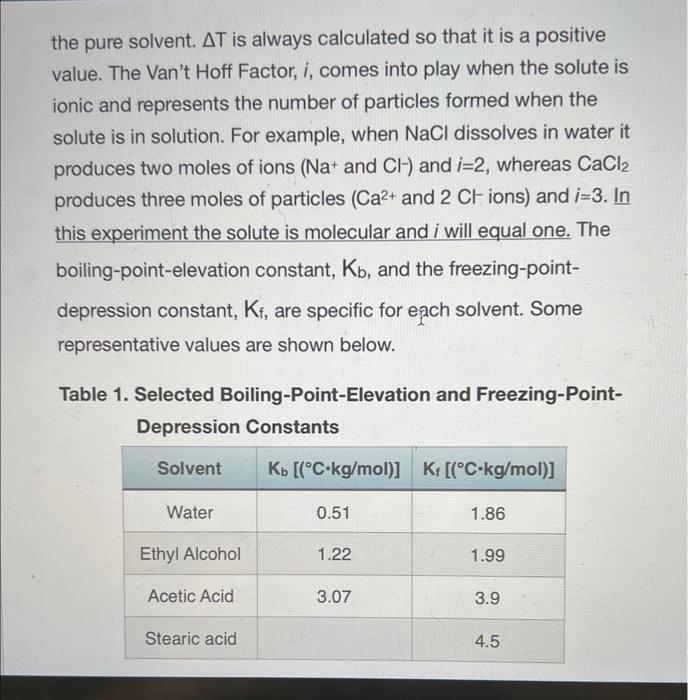
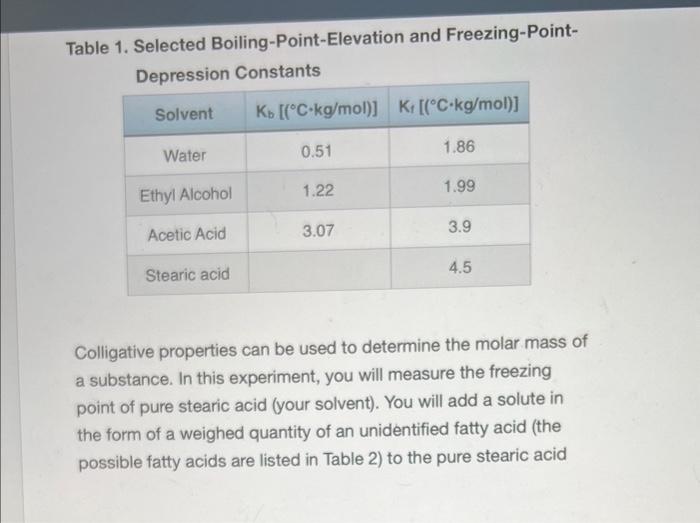
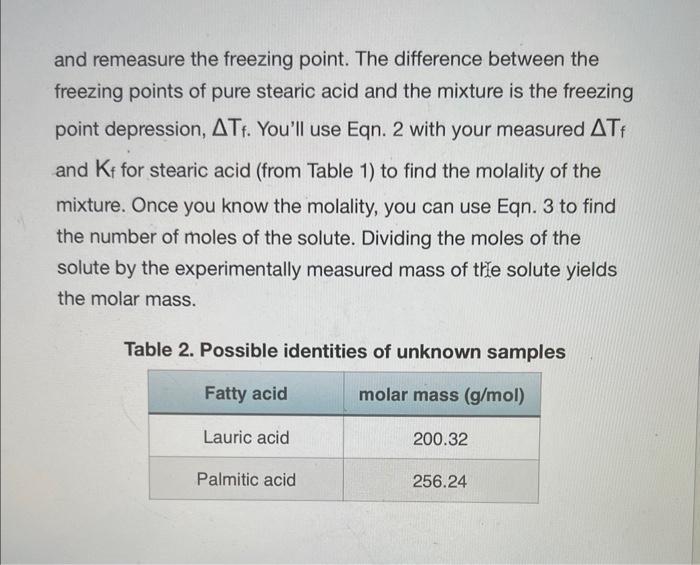
Calculations: 1. Separately calculate the molar mass of your unknown acid for mixtures 1 and 2 (be careful - the grams of unknown acid for mixture 2 is approximately 2g because it is the total mass of the unknown acid added to make mixture 1 and mixture 2): molarmass=molesunknownacidgramsunknownacid 2. Calculate the average molar mass. 3. Using the data from Table 2, determine the identity of your unknown acid and calculate a percent error in molar mass. Table 2. Possible identities of unknown samples When a nonvolatile solute is dissolved in a solvent, the solvent's boiling point increases, its freezing point decreases, and its osmotic pressure increases. These characteristics of a solution are termed the colligative properties. Colligative effects are observed in many phenomena such as the melting of ice on winter roads from "salting", addition of antifreeze to a car's radiator to prevent "freeze up", and the rising of water in trees due to osmotic pressure. Interestingly, colligative effects, such as freezing point depression and boiling point elevation, only depend upon the number of particles dissolved in the solute and not on the identity of the solvent. The relationships between the elevation in boiling point (Tb) and the depression in freezing point (Tf) to the concentration of solute expressed in molality (m) are: Tb=KbmiTf=Kfmi Eqn. 1 Eqn. 2 where molality is defined as the number of moles of solute per kilogram of solvent: m=kgsolventnsolute Eqn. 3 Tb is the difference between the boiling points of the solution and the pure solvent, and Tf is the difference between the freezing points of the solution and the pure solvent. T is always calculated so that it is a positive value. The Van't Hoff Factor, i, comes into play when the solute is ionic and represents the number of particles formed when the solute is in solution. For example, when NaCl dissolves in water it produces two moles of ions (Na+and Cl)and i=2, whereas CaCl2 produces three moles of particles ( Ca2+ and 2Clions) and i3. In this experiment the solute is molecular and i will equal one. The boiling-point-elevation constant, Kb, and the freezing-pointdepression constant, Kf, are specific for each solvent. Some representative values are shown below. Table 1. Selected Boiling-Point-Elevation and Freezing-PointDepression Constants Colligative properties can be used to determine the molar mass of a substance. In this experiment, you will measure the freezing point of pure stearic acid (your solvent). You will add a solute in the form of a weighed quantity of an unidentified fatty acid (the possible fatty acids are listed in Table 2) to the pure stearic acid and remeasure the freezing point. The difference between the freezing points of pure stearic acid and the mixture is the freezing point depression, Tf. You'll use Eqn. 2 with your measured Tf and Kf for stearic acid (from Table 1) to find the molality of the mixture. Once you know the molality, you can use Eqn. 3 to find the number of moles of the solute. Dividing the moles of the solute by the experimentally measured mass of thle solute yields the molar mass. Table 2. Possible identities of unknown samples
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


