Question: please do 3rd and 4th question 1. Using Excel, make a plot of the vapor temperature vs. volume for the 15 samples. Follow the graphing
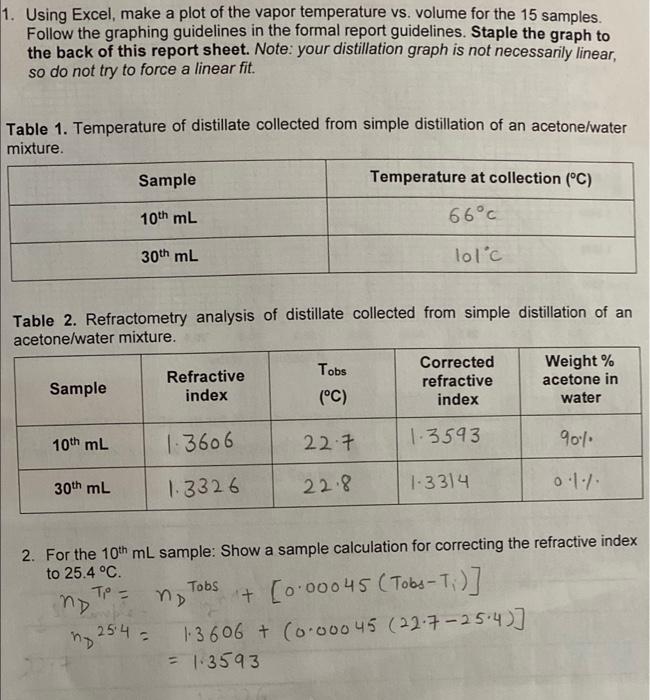
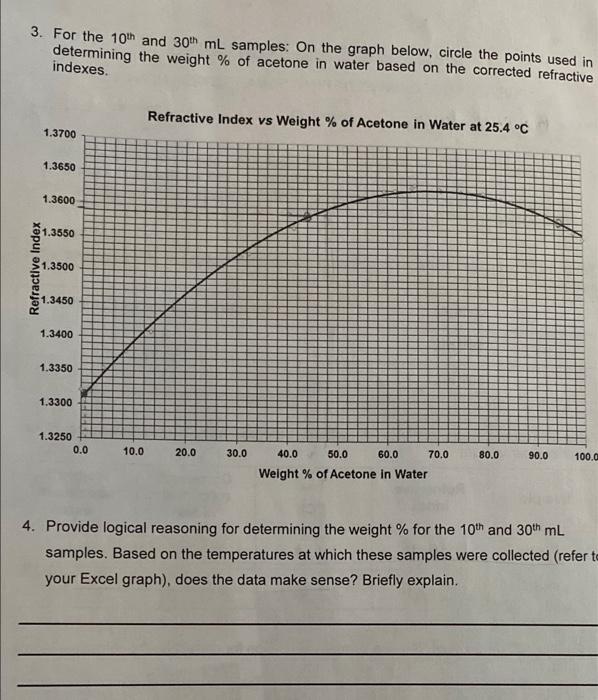
1. Using Excel, make a plot of the vapor temperature vs. volume for the 15 samples. Follow the graphing guidelines in the formal report guidelines. Staple the graph to the back of this report sheet. Note: your distillation graph is not necessarily linear, so do not try to force a linear fit. Table 1. Temperature of distillate collected from simple distillation of an acetone/water mixture. Sample Temperature at collection (C) 10th mL 66C 30th mL lolc Table 2. Refractometry analysis of distillate collected from simple distillation of an acetone/water mixture. Refractive index Corrected refractive index Tobs (C) Sample Weight % acetone in water 10th mL 1.3606 22.7 1.3593 900 30th mL 1.3326 22.8 1.3314 01. 2. For the 10th mL sample: Show a sample calculation for correcting the refractive index to 25.4 C Tobs + [0.00045 (Tobs-7:)] 1-3 606 + (0-00045 (22-7-25:4)] = 1.3593 To = no 254 no 3. For the 10th and 30th mL samples: On the graph below, circle the points used in determining the weight % of acetone in water based on the corrected refractive indexes Refractive Index vs Weight % of Acetone in Water at 25.4 C 1.3700 1.3650 1.3600 1.3550 Refractive Index 1.3500 51.3450 1.3400 1.3350 1.3300 1.3250 0.0 10.0 20.0 30.0 80.0 90.0 100.0 40.0 50.0 60.0 70.0 Weight % of Acetone in Water 4. Provide logical reasoning for determining the weight % for the 10th and 30th mL samples. Based on the temperatures at which these samples were collected (refer to your Excel graph), does the data make sense? Briefly explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


