Question: please do all 3. thank you:) Ramal Devise the most efficient synthesis for the carboxylic acid below using a starting material and reagents from the

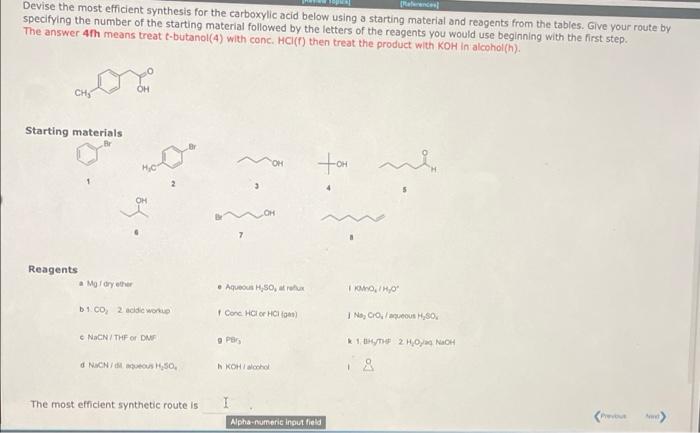
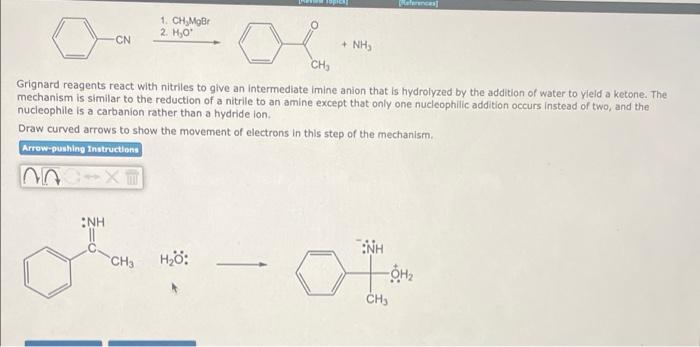
Ramal Devise the most efficient synthesis for the carboxylic acid below using a starting material and reagents from the tables. Give your route by specifying the number of the starting material followed by the letters of the reagents you would use beginning with the first step. The answer 4th means treat e-butanol(4) with conc. HCIC) then treat the product with KOH in alcohol(!). recor OH Starting materials tom 4 OH Reagents a Mary ether Aqueous 30. rux KM/H, b co, 2.0d work I Conc HC HCI) Na Colaus H 30 NOCN/THEO DE 9 Per * 1 BHYTHE 2 HOH d NacNH, 50 KOH/cohol The most efficient synthetic route is Devise the most efficient synthesis for the carboxylic acid below using a starting material and reagents from the tables. Give your route by specifying the number of the starting material followed by the letters of the reagents you would use beginning with the first step. The answer 4th means treat e-butanol(4) with conc. HCI(T) then treat the product with KOH in alcohol(h). OH Starting materials OH tom 3 4 OH Reagents a Modryer Aqueous H,SO, OTHO 1.CO 2 acidic w Cone HC HCH) IN, CO:/out 7.30 1, 2 NaCN/THF OF DAF 9 PB NICNIH 50 - KH tr The most efficient synthetic route is I Alphanumeric input field 1. CH, MOB 2 H, CN + NH3 CH, Grignard reagents react with nitriles to give an intermediate imine anion that is hydrolyzed by the addition of water to yield a ketone. The mechanism is similar to the reduction of a nitrile to an amine except that only one nucleophilic addition occurs instead of two, and the nucleophile is a carbanlon rather than a hydride ion. Draw curved arrows to show the movement of electrons in this step of the mechanism Arrow.pushing Instructions NH Z=0 CH3 H: INH OH CH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


