Question: please do all or most For a frut order reacson, the initial reactant concentroon is 0.20M. After 12.5 s the concentration is 0.17M. What is
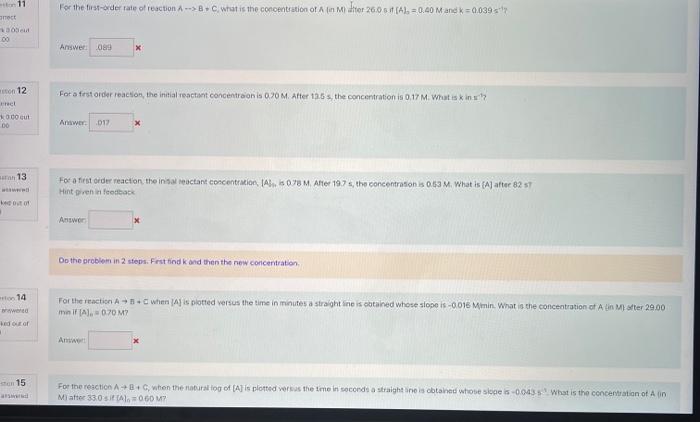
For a frut order reacson, the initial reactant concentroon is 0.20M. After 12.5 s the concentration is 0.17M. What is k in sth, For a first order reacton, the insal reactant coscentruion, [A], is 07sM, After 19.7s, the concentrason is 0.63M, What is [A] after 82 s) Hint pren it feotleack Asinver Do the problem in 2 steps. Frst find k and then the new concentration. mn if [A]e=0.00M ? Miatter 33.0 sif (A)0=060Mr
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


