Question: PLEASE DO ALL THE PARTS and DO Not Copy and paste from Chegg. Experiment-A9: Heat Capacity and Enthalpy of Dissolution Objectives: In this experiment you
PLEASE DO ALL THE PARTS and DO Not Copy and paste from Chegg. 


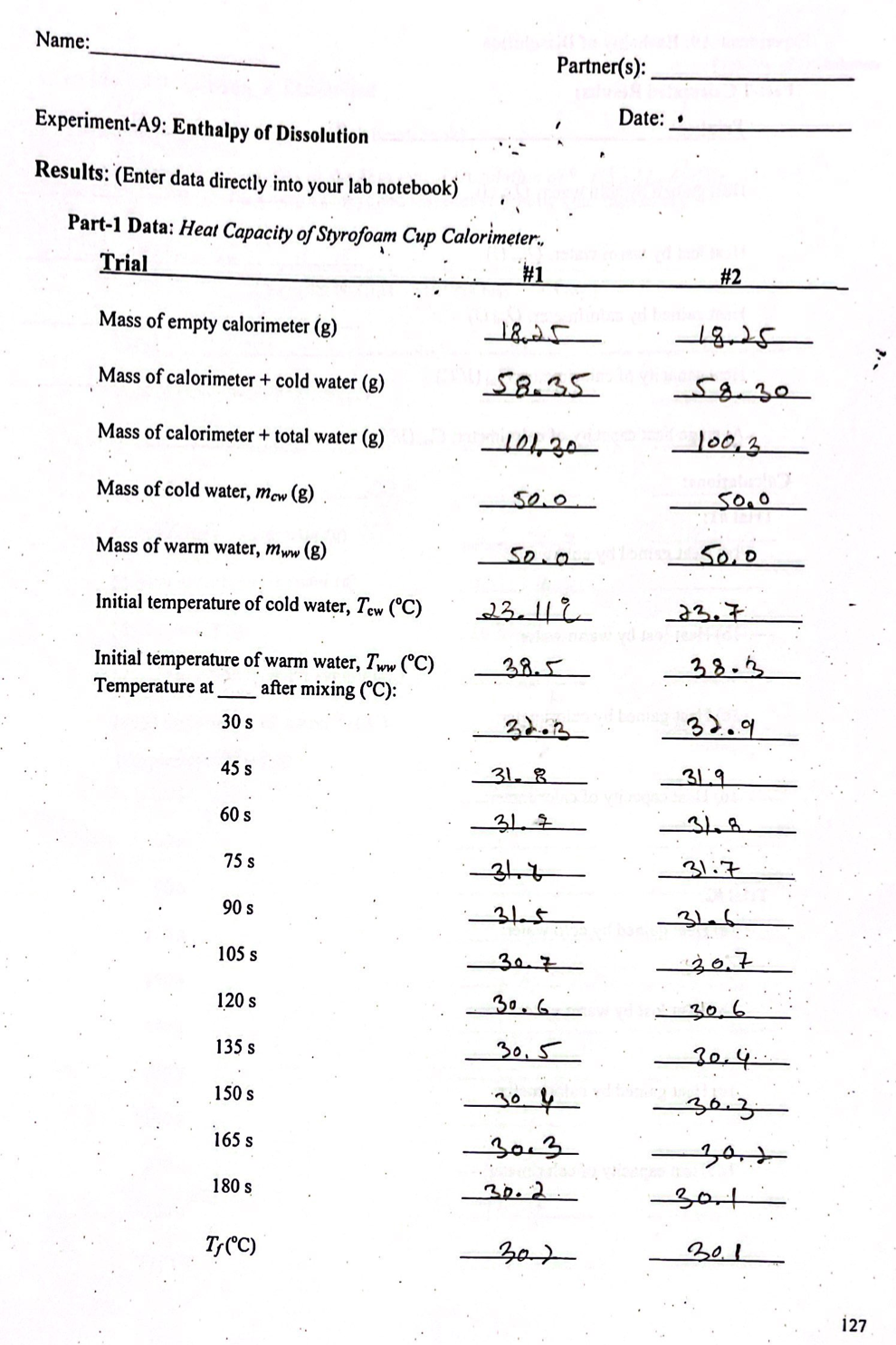

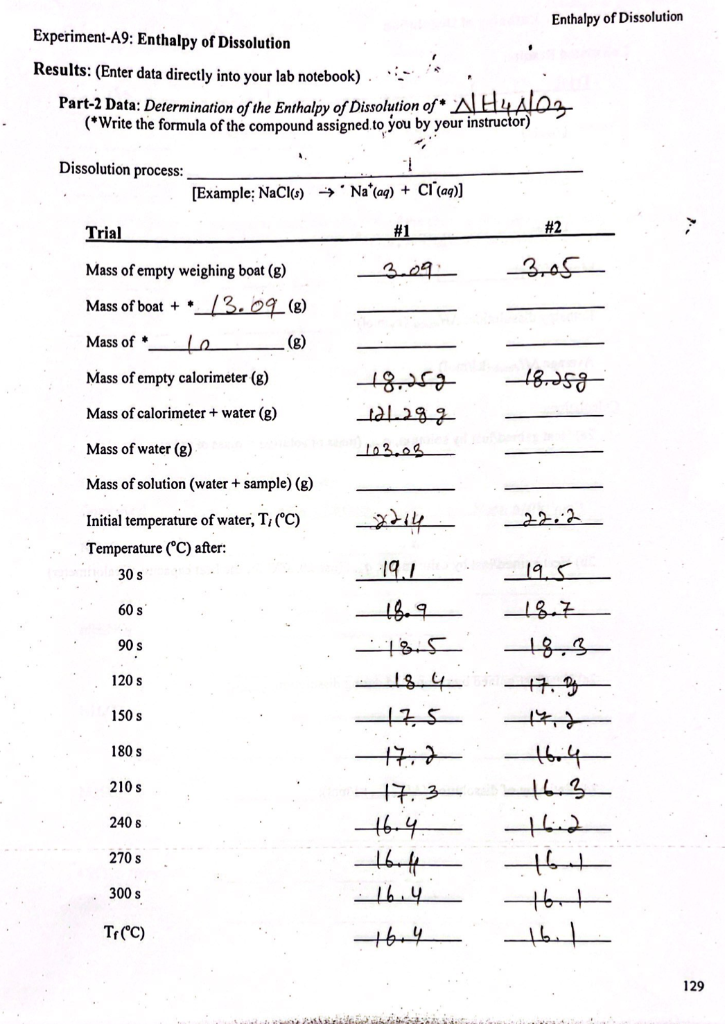

Experiment-A9: Heat Capacity and Enthalpy of Dissolution Objectives: In this experiment you will determine the heat capacity of a calorimeter and the heat of dissolution of several ionic compounds in water. Concept of the experiment Most chemical reactions are accompanied by energy changes that result in heat being released or absorbed. Chemical reactions that release heat are called exothermic reactions and those that absorb heat are called endothermic reactions. Some reactions produce so much heat that they result in a flame and/or explosion. The dissolution (dissolving) of an ionic compound can be regarded as a chemical process, because it involves separation of ions and interactions of ions with water. For example, when sodium chloride is dissolved in water, the following process takes place: NaCl(s) + excess HO(0) Nat(aq) + Cl (aq); (eqn.1) Step-1: NaCl(s) (eqn.la) This dissolution process can be broken down into two steps: Na*(g) + Cl (g); . Step-2: Na (g) + Cl (g) + excess HO(1) Na*(aq) + Cl(aq); Overall: NaCl(s) + excess HO() Na* (aq) + Cl(aq); AH soln where AH dissoln. = AHlat + AHhyd AHhyd. (egn.1b) (eqn.1c) Step-1 is an endothermic process (AHat> 0); heat is required to separate ions from a solid ionic compound. While Step-2 is an exothermic process (AHhyd
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


