Question: please do all three problems What is the molarity of a solution of calcium hydroxide, Ca(OH), if 339 mi is neutralized with 4151 ml of
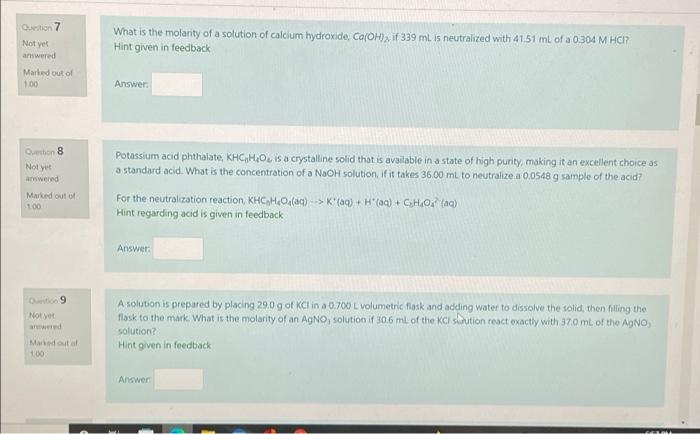
What is the molarity of a solution of calcium hydroxide, Ca(OH), if 339 mi is neutralized with 4151 ml of a 0,304 M HCI? Hint given in feedback Question 7 Not yet answered Marked out of 100 Answer Question 8 Not yet answered Marked out of 100 Potassium acid phthalate, KHC,H,O, is a crystalline solid that is available in a state of high purity, making it an excellent choice as a standard acid. What is the concentration of a NaOH solution. If it takes 36.00 mL to neutralize a 0.0548 g sample of the acid? For the neutralization reaction KHCHO (aq) --> K"(aq) + H*(aq) + CHO, (a) Hint regarding acid is given in feedback Answer: 9 Note A solution is prepared by placing 29.0 g of KCl in a 0.700 L volumetric flosk and adding water to dissolve the solid, then filling the flask to the mark. What is the molarity of an AgNO, solution if 30.6 mL of the ci slution react exactly with 370 mL of the AgNO, solution? Hint given in feedback Mostal 100
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


