Question: please do calculations that you can without experimental data Experiment 2: Partition coefficient and transfer free energy Background: As you saw in the previous lab,


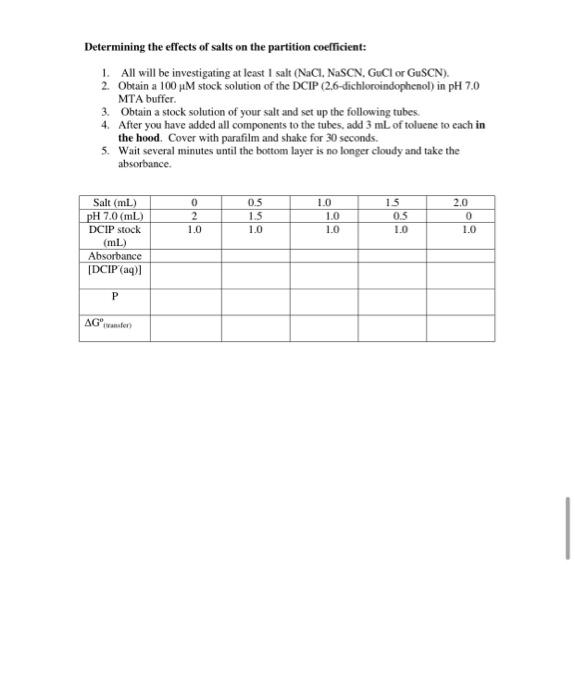
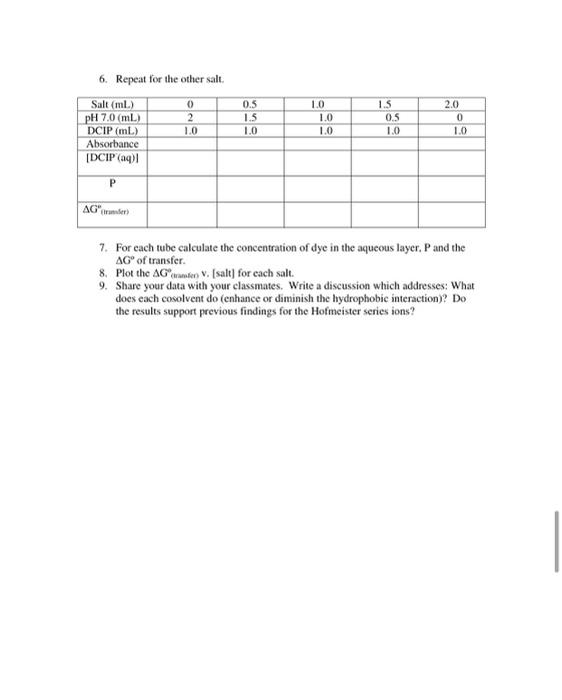
Experiment 2: Partition coefficient and transfer free energy Background: As you saw in the previous lab, different molecules have different degrees of hydrophobicity. In general, those composed of primarily aliphatic and aromatic groups tend to be hydrophobic, whereas those with charged groups or many hydrogen bond donors and acceptors tend to be more hydrophilic. In addition to the structure of the molecule of interest, it is also known that adding salts to the aqueous layer will also influence the extent to which a hydrophobic molecule will partition into the aqueous layer. Some ions are able to enhance the solubility of hydrophobic molecules in water (.e. "salt in"), whereas some diminish the solubility of hydrophobic molecules in water Ci.e. "salt out"). This effect was first discovered over 100 years ago by Franz Hofmeister, and so these ions are referred to as the Hofmeister series. The Hofmeister series is shown below: Enhance hydrophobic interactions- ---Diminish hydrophobie interactions NH, >K'>Na > Li > Mg** > Ca>Guanidinium (Gus) so, > HPO > AcO > citrate > tartrate > C > NOK > CIO >SCN DCIP (2,6-dichloroindophenol) has two ionic forms, the neutral, protonated form (DCIPH) which predominates under acidic conditions, and the negatively charged form (DCIP) which predominates under neutral and basic conditions as shown below: + H(aq) OH DCPH (tol) DCIPH( DCP (a) red red blue In the previous experiment, you most likely) observed that the presence of charge on a molecule tends to make it hydrophilic regardless of the rest of the structure. Therefore, it is reasonable to assume that it is primarily the neutral form of DCIP that partitions into the toluene layer. We can consider the following equilibrium for the biphasic mixture of toluene (the organic solvent in this experiment) and aqueous DCIP solutions: DCIPH(tol) DCIPH(aq) DCIP(aq) + H(aq) The first equilibrium is governed by the partition coefficient, P. P [DCIPH(tol)] (DCIPH(20) The second equilibrium is governed by the acid dissociation constant of DCIP, K, which has a value of 1.25 x 10 shown in equation 9. (8) (DCIP(q)][H+ (aq)] (9) [DCIPH(aq)] Thus the overall process is governed by the following equilibrium expression: (DCIPH(tol)] (10) KADCIP-(aq)][H*(aq)] Since both the aqueous and the toluene layer have the same volume we can calculate the concentration of the DCIP in the toluene layer using equation 11 as follows: (DCIP = IDCIPH(tol)) + (DCIPH(aq)] + [DCIP (aq) Thus, combining the equilibrium constants in equations 8, 9 and 10 with the mass balance equation 11, we can calculate the partition coefficient from the measured concentration of DCIP in the aqueous layer, the known initial concentration of DCIP, and the known concentration of H+, as follows in equation 12. P = Ka DCIP]initial - (DCIP(aq)]:H*1- [DOP(aq)] Ke (12) (DCIP )) (H+) To determine the partition coefficient, you will be measuring the concentration of dye in water ([DCIP)uter). In order to do this you will need to use the Beer-Lambert Law: A = sel where A is the absorbance E is the molar extinction coefficient c is the concentration of the dye I is the pathlength which is just lcm in our case We previously determined E008mm = 8100 M em for DCIP at pH 7.0. Determining the effects of salts on the partition coefficient: 1. All will be investigating at least 1 salt (NaCl, NaSCN. GuCl or GuSCN). 2. Obtain a 100M stock solution of the DCIP (2.6-dichloroindophenol) in pH 7.0 MTA buffer. 3. Obtain a stock solution of your salt and set up the following tubes 4. After you have added all components to the tubes, add 3 mL of toluene to each in the hood. Cover with parafilm and shake for 30 seconds. 5. Wait several minutes until the bottom layer is no longer cloudy and take the absorbance. 0 2 1.0 0.5 15 1.0 1.0 1.0 1.0 15 0.5 1.0 2.0 0 1.0 Salt (ml) pH 7.0(mL) DCIP stock (ml) Absorbance [DCIP(aq)] P AGwen 6. Repeat for the other salt Salt (ml) 0 pH 7.0 (mL) 2 DCIP (mL) 1.0 Absorbance (DCIP(aq) 0.5 1.5 10 10 1.0 1.0 1.5 OS 1.0 2.0 0 1.0 P AG 7. For each tube calculate the concentration of dye in the aqueous layer. P and the AG of transfer. 8. Plot the AG a V. (salt) for each salt. 9. Share your data with your classmates. Write a discussion which addresses: What does each cosolvent do (enhance or diminish the hydrophobic interaction)? Do the results support previous findings for the Hofmeister series ions? Experiment 2: Partition coefficient and transfer free energy Background: As you saw in the previous lab, different molecules have different degrees of hydrophobicity. In general, those composed of primarily aliphatic and aromatic groups tend to be hydrophobic, whereas those with charged groups or many hydrogen bond donors and acceptors tend to be more hydrophilic. In addition to the structure of the molecule of interest, it is also known that adding salts to the aqueous layer will also influence the extent to which a hydrophobic molecule will partition into the aqueous layer. Some ions are able to enhance the solubility of hydrophobic molecules in water (.e. "salt in"), whereas some diminish the solubility of hydrophobic molecules in water Ci.e. "salt out"). This effect was first discovered over 100 years ago by Franz Hofmeister, and so these ions are referred to as the Hofmeister series. The Hofmeister series is shown below: Enhance hydrophobic interactions- ---Diminish hydrophobie interactions NH, >K'>Na > Li > Mg** > Ca>Guanidinium (Gus) so, > HPO > AcO > citrate > tartrate > C > NOK > CIO >SCN DCIP (2,6-dichloroindophenol) has two ionic forms, the neutral, protonated form (DCIPH) which predominates under acidic conditions, and the negatively charged form (DCIP) which predominates under neutral and basic conditions as shown below: + H(aq) OH DCPH (tol) DCIPH( DCP (a) red red blue In the previous experiment, you most likely) observed that the presence of charge on a molecule tends to make it hydrophilic regardless of the rest of the structure. Therefore, it is reasonable to assume that it is primarily the neutral form of DCIP that partitions into the toluene layer. We can consider the following equilibrium for the biphasic mixture of toluene (the organic solvent in this experiment) and aqueous DCIP solutions: DCIPH(tol) DCIPH(aq) DCIP(aq) + H(aq) The first equilibrium is governed by the partition coefficient, P. P [DCIPH(tol)] (DCIPH(20) The second equilibrium is governed by the acid dissociation constant of DCIP, K, which has a value of 1.25 x 10 shown in equation 9. (8) (DCIP(q)][H+ (aq)] (9) [DCIPH(aq)] Thus the overall process is governed by the following equilibrium expression: (DCIPH(tol)] (10) KADCIP-(aq)][H*(aq)] Since both the aqueous and the toluene layer have the same volume we can calculate the concentration of the DCIP in the toluene layer using equation 11 as follows: (DCIP = IDCIPH(tol)) + (DCIPH(aq)] + [DCIP (aq) Thus, combining the equilibrium constants in equations 8, 9 and 10 with the mass balance equation 11, we can calculate the partition coefficient from the measured concentration of DCIP in the aqueous layer, the known initial concentration of DCIP, and the known concentration of H+, as follows in equation 12. P = Ka DCIP]initial - (DCIP(aq)]:H*1- [DOP(aq)] Ke (12) (DCIP )) (H+) To determine the partition coefficient, you will be measuring the concentration of dye in water ([DCIP)uter). In order to do this you will need to use the Beer-Lambert Law: A = sel where A is the absorbance E is the molar extinction coefficient c is the concentration of the dye I is the pathlength which is just lcm in our case We previously determined E008mm = 8100 M em for DCIP at pH 7.0. Determining the effects of salts on the partition coefficient: 1. All will be investigating at least 1 salt (NaCl, NaSCN. GuCl or GuSCN). 2. Obtain a 100M stock solution of the DCIP (2.6-dichloroindophenol) in pH 7.0 MTA buffer. 3. Obtain a stock solution of your salt and set up the following tubes 4. After you have added all components to the tubes, add 3 mL of toluene to each in the hood. Cover with parafilm and shake for 30 seconds. 5. Wait several minutes until the bottom layer is no longer cloudy and take the absorbance. 0 2 1.0 0.5 15 1.0 1.0 1.0 1.0 15 0.5 1.0 2.0 0 1.0 Salt (ml) pH 7.0(mL) DCIP stock (ml) Absorbance [DCIP(aq)] P AGwen 6. Repeat for the other salt Salt (ml) 0 pH 7.0 (mL) 2 DCIP (mL) 1.0 Absorbance (DCIP(aq) 0.5 1.5 10 10 1.0 1.0 1.5 OS 1.0 2.0 0 1.0 P AG 7. For each tube calculate the concentration of dye in the aqueous layer. P and the AG of transfer. 8. Plot the AG a V. (salt) for each salt. 9. Share your data with your classmates. Write a discussion which addresses: What does each cosolvent do (enhance or diminish the hydrophobic interaction)? Do the results support previous findings for the Hofmeister series ions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


