Question: .. please do it fast.. The reaction for the conversion of sulfate to H2S(g) may be written: SO4 + CH3COOH + 2H+ H2S(g) + 2H2O
.. please do it fast..
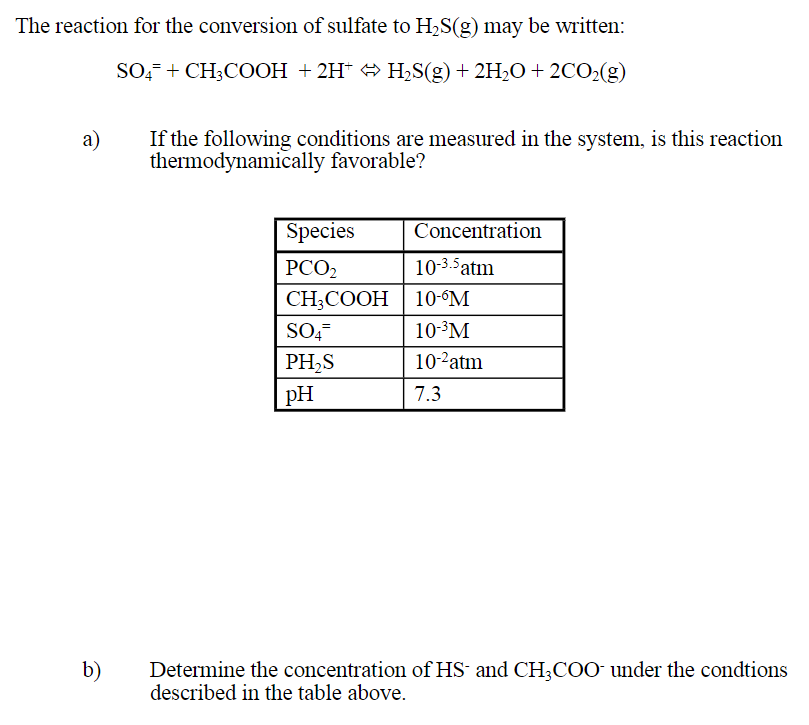
The reaction for the conversion of sulfate to H2S(g) may be written: SO4 + CH3COOH + 2H+ H2S(g) + 2H2O + 2CO2(g) a) If the following conditions are measured in the system, is this reaction thermodynamically favorable? Species Concentration PCO2 10-3.5 atm CH3COOH | 10-M SO4- 10PM PHS 10-2atm PH 7.3 b) Determine the concentration of HS and CH3COO under the condtions described in the table above
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


