Question: please do it quickly !!!!!!!!!!!!!!!!!! To study the adsorption of charcoal in Acetic acid (standardized 0.380M), charcoal was added to bottles containing different volumes of
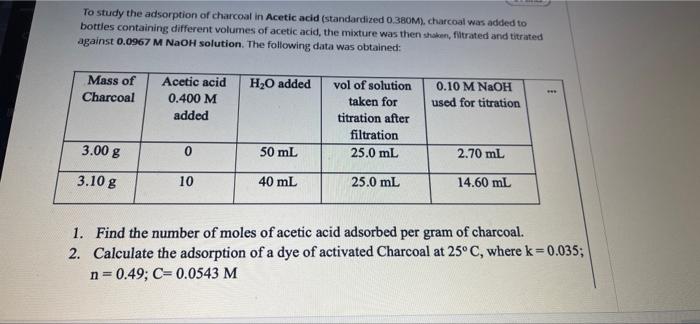
To study the adsorption of charcoal in Acetic acid (standardized 0.380M), charcoal was added to bottles containing different volumes of acetic acid, the mixture was then shake, filtrated and titrated against 0.0967 M NaOH solution. The following data was obtained: Mass of Charcoal H20 added Acetic acid 0.400 M added 0.10 M NaOH used for titration vol of solution taken for titration after filtration 25.0 mL 3.008 0 50 mL 2.70 mL 3.10 g 10 40 mL 25.0 mL 14.60 ml 1. Find the number of moles of acetic acid adsorbed per gram of charcoal. 2. Calculate the adsorption of a dye of activated Charcoal at 25C, where k=0.035; n=0.49; C= 0.0543 M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


