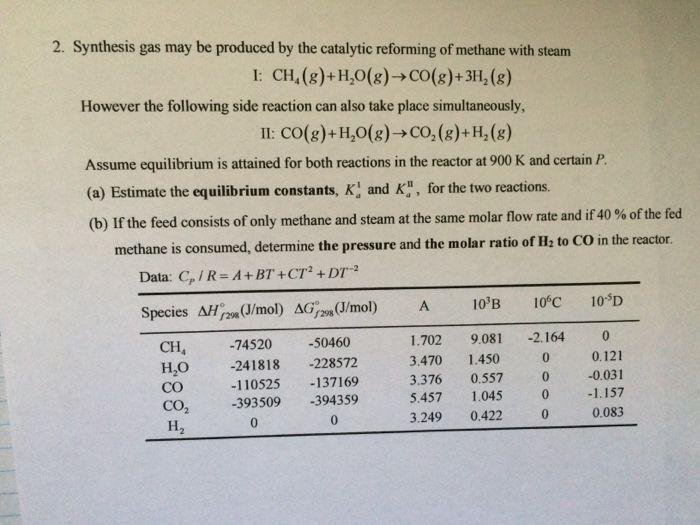
Question: Please do not copy 2. Synthesis gas may be produced by the catalytic reforming of methane with steam 1: CH.(8)+H30(8) CO(g)+3H (8) However the following
 Please do not copy
Please do not copy
2. Synthesis gas may be produced by the catalytic reforming of methane with steam 1: CH.(8)+H30(8) CO(g)+3H (8) However the following side reaction can also take place simultaneously, II: CO(g)+H2O(g) CO2(g)+H,(8) Assume equilibrium is attained for both reactions in the reactor at 900 K and certain P. (a) Estimate the equilibrium constants, K. and K.", for the two reactions (b) If the feed consists of only methane and steam at the same molar flow rate and if 40% of the fed methane is consumed, determine the pressure and the molar ratio of Hz to CO in the reactor. Data: C/R=A+BT +CT2 + DT-2 Species AH 298 (I/mol) AG29 (J/mol) A 10 B 10C 10D CH, ,0 CO CO2 H, -74520 -241818 -110525 -393509 0 -50460 -228572 -137169 -394359 0 1.702 3.470 3.376 5.457 3.249 9.081 1.450 0.557 1.045 0.422 -2.164 0 0 0 0 0.121 -0.031 -1.157 0.083 0 2. Synthesis gas may be produced by the catalytic reforming of methane with steam 1: CH.(8)+H30(8) CO(g)+3H (8) However the following side reaction can also take place simultaneously, II: CO(g)+H2O(g) CO2(g)+H,(8) Assume equilibrium is attained for both reactions in the reactor at 900 K and certain P. (a) Estimate the equilibrium constants, K. and K.", for the two reactions (b) If the feed consists of only methane and steam at the same molar flow rate and if 40% of the fed methane is consumed, determine the pressure and the molar ratio of Hz to CO in the reactor. Data: C/R=A+BT +CT2 + DT-2 Species AH 298 (I/mol) AG29 (J/mol) A 10 B 10C 10D CH, ,0 CO CO2 H, -74520 -241818 -110525 -393509 0 -50460 -228572 -137169 -394359 0 1.702 3.470 3.376 5.457 3.249 9.081 1.450 0.557 1.045 0.422 -2.164 0 0 0 0 0.121 -0.031 -1.157 0.083 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


