Question: please do not copy random answers! That will be thumbs down! 2) Consider the separation of singly charged ions of U-238 and U-235 isotopes with
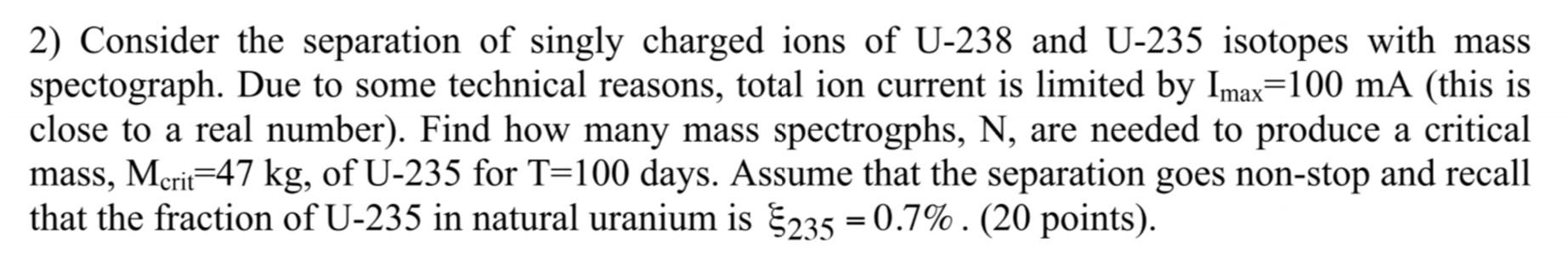 please do not copy random answers! That will be thumbs down!
please do not copy random answers! That will be thumbs down!
2) Consider the separation of singly charged ions of U-238 and U-235 isotopes with mass spectograph. Due to some technical reasons, total ion current is limited by Imax=100 mA (this is close to a real number). Find how many mass spectrogphs, N, are needed to produce a critical mass, Mcrit=47 kg, of U-235 for T=100 days. Assume that the separation goes non-stop and recall that the fraction of U-235 in natural uranium is $235 = 0.7%. (20 points). ==
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


