Question: please do parts d and e and check a if you can! 2ADPATP+AMP:H=2.02kJ/mol and S=0.0242kJ/molK. a.) ( 3 pts) Based on the information given above,
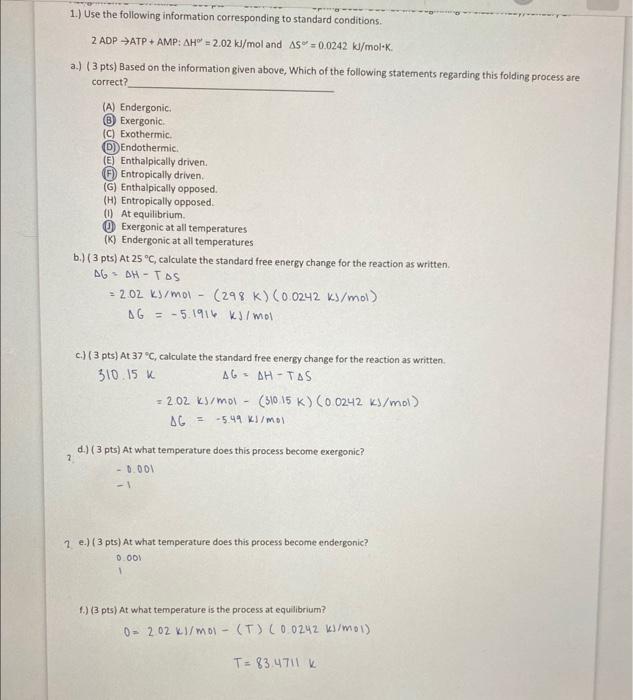
2ADPATP+AMP:H=2.02kJ/mol and S=0.0242kJ/molK. a.) ( 3 pts) Based on the information given above, Which of the following statements regarding this folding process are correct? (A) Endergonic. (B) Exergonic. (C) Exothermic. (D) Endothermic. (E) Enthalpically driven. (F) Entropically driven. (G) Enthalpically opposed. (H) Entropically opposed. (i) At equilibrium. (D) Exergonic at all temperatures (K) Endergonic at all temperatures b.) ( 3pts) At 25C, calculate the standard free energy change for the reaction as written. G=G=HTS2.02kJ/mol(298K)(0.0242kJ/mol)=5.1916kJ/mol c.) ( 3 pts) At 37C, calculate the standard free energy change for the reaction as written. 310.15KG=G==HTS2.02ks/mol(310.15K)(0.0242kJ/mol)5.49ks/mol d.) ( 3 pts) At what temperature does this process become exergonic? 0.0011 2. e.) ( 3 pts) At what temperature does this process become endergonic? 0.001 1.) (3 pts) At what temperature is the process at equilibrium? 0=2.02kl/mol(T)(0.0242k)/mol)T=83.4711k
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


