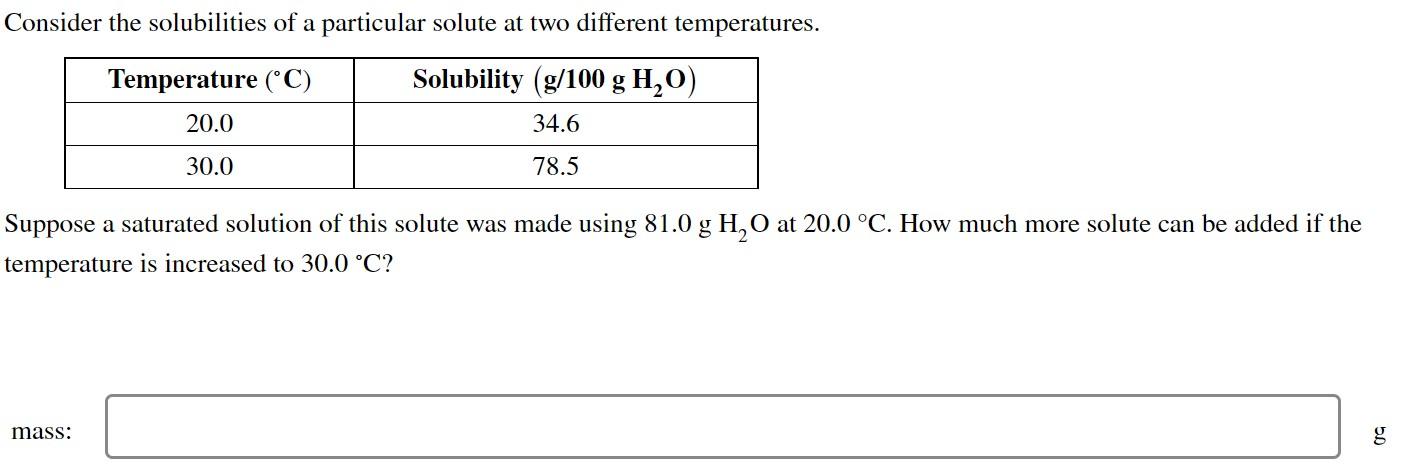
Question: Please do step by step with good explanation! Consider the solubilities of a particular solute at two different temperatures. Suppose a saturated solution of this
 Please do step by step with good explanation!
Please do step by step with good explanation!
Consider the solubilities of a particular solute at two different temperatures. Suppose a saturated solution of this solute was made using 81.0gH2O at 20.0C. How much more solute can be added if the temperature is increased to 30.0C ? mass
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


