Question: please do the labeling in the graph provided 7.) (15pts) Consider the two-component liquid-vapor phase diagram shown where pressure is held constant (Making the phase
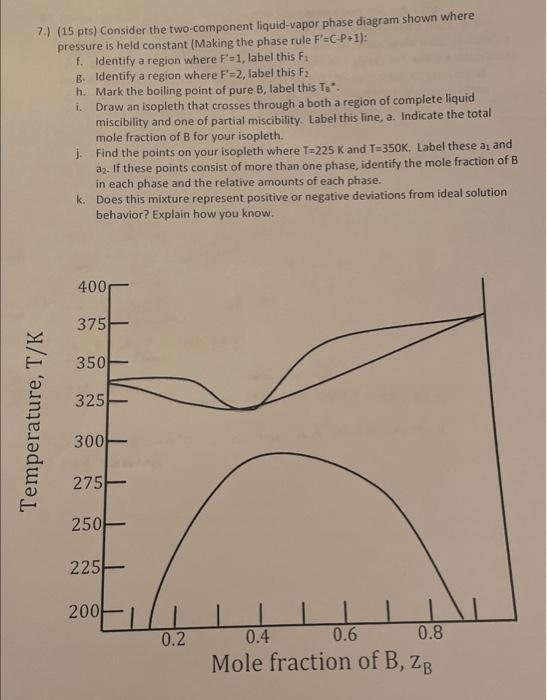
7.) (15pts) Consider the two-component liquid-vapor phase diagram shown where pressure is held constant (Making the phase rule F=CP+1 ): f. Identify a region where F=1, label this F1 B. Identify a region where F=2, label this F2 h. Mark the boiling point of pure B, label this Ta. i. Draw an isopleth that crosses through a both a region of complete liquid miscibility and one of partial miscibility. Label this line, a. Indicate the total mole fraction of B for your isopleth. j. Find the points on your isopleth where T=225K and T=350K. Label these a1 and a2. If these points consist of more than one phase, identify the mole fraction of B in each phase and the relative amounts of each phase. k. Does this mixture represent positive or negative deviations from ideal solution behavior? Explain how you know
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


