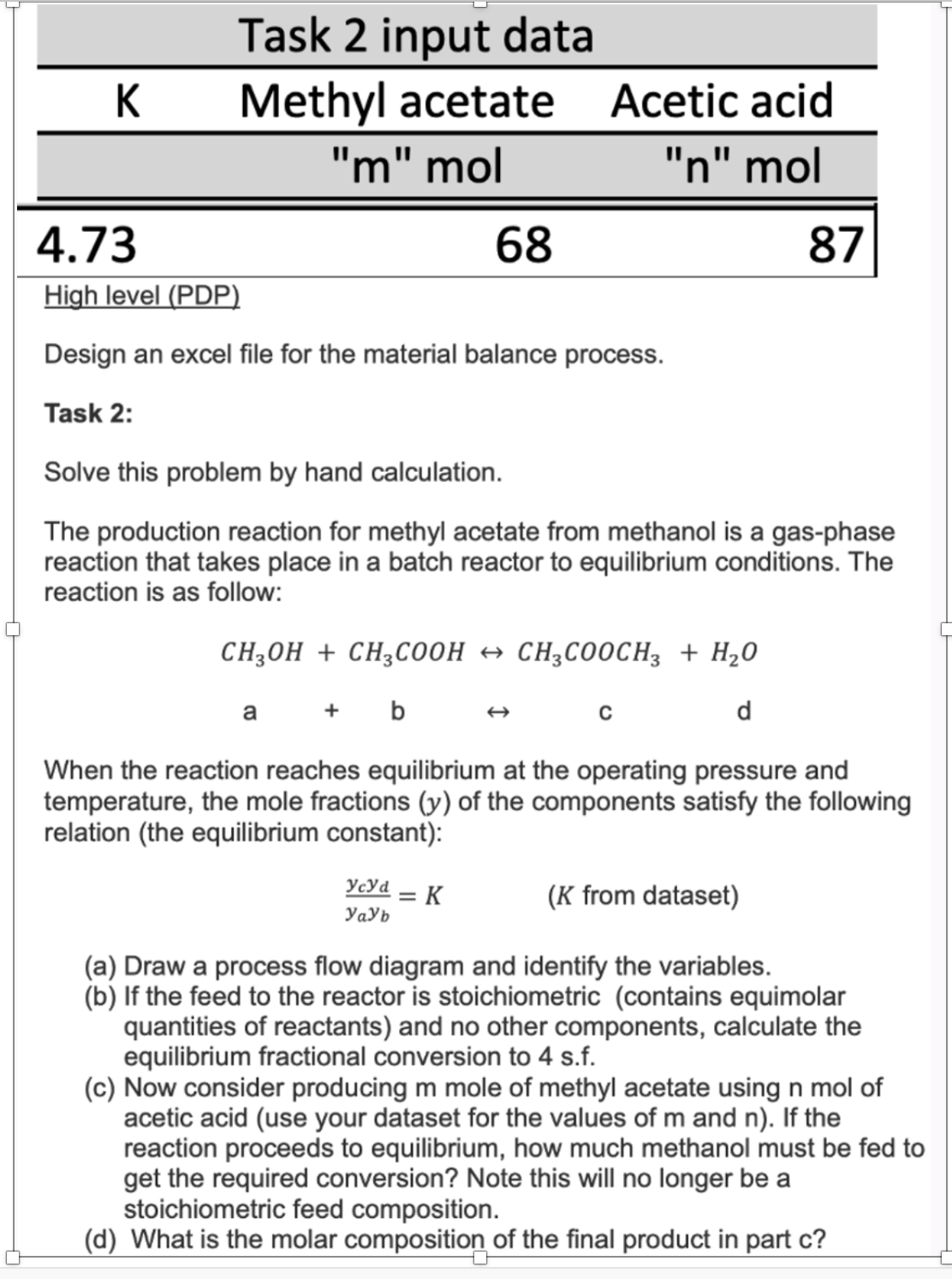
Question: Please do this and answer all qustions thank you. Design an excel file for the material balance process. Task 2 : Solve this problem by
Please do this and answer all qustions thank you.
Design an excel file for the material balance process.
Task :
Solve this problem by hand calculation.
The production reaction for methyl acetate from methanol is a gasphase
reaction that takes place in a batch reactor to equilibrium conditions. The
reaction is as follow:
harr
When the reaction reaches equilibrium at the operating pressure and
temperature, the mole fractions of the components satisfy the following
relation the equilibrium constant:
from dataset
a Draw a process flow diagram and identify the variables.
b If the feed to the reactor is stoichiometric contains equimolar
quantities of reactants and no other components, calculate the
equilibrium fractional conversion to f
c Now consider producing mole of methyl acetate using mol of
acetic acid use your dataset for the values of and If the
reaction proceeds to equilibrium, how much methanol must be fed to
get the required conversion? Note this will no longer be a
stoichiometric feed composition.
d What is the molar composition of the final product in part c K methyl acetate m mol, acetic acid n mol
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


