Question: Please do this in MATLAB, thank you 1. The Carnot engine is an ideal cyclic heat engine that gives the maximum amount of work. A
Please do this in MATLAB, thank you
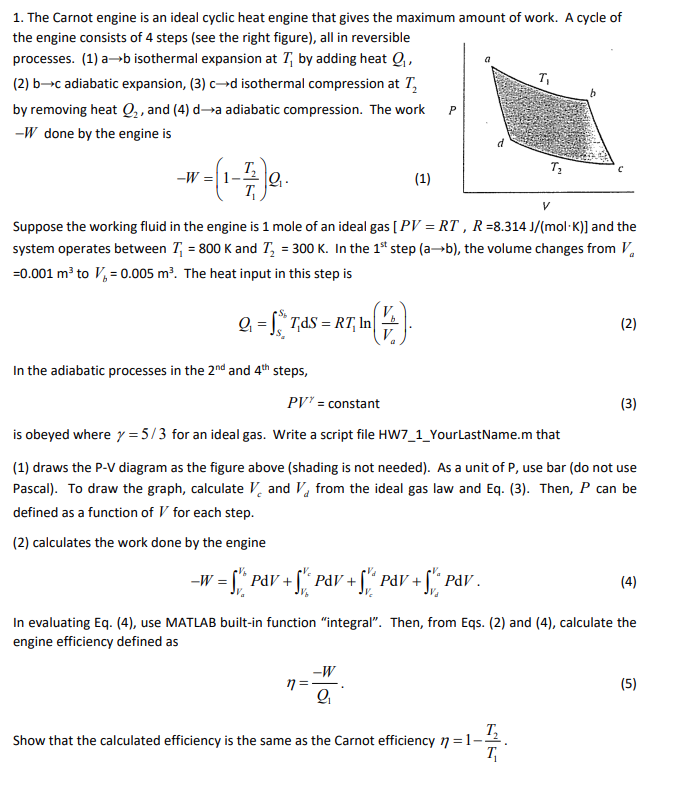
1. The Carnot engine is an ideal cyclic heat engine that gives the maximum amount of work. A cycle of the engine consists of 4 steps (see the right figure), all in reversible processes. (1) ab isothermal expansion at T by adding heat Q (2) b-c adiabatic expansion, (3) cd isothermal compression at I, by removing heat Q2, and (4) da adiabatic compression. The work -W done by the engine is T P d T: -W = 1- -. 2 (1) V ) Suppose the working fluid in the engine is 1 mole of an ideal gas [PV = RT , R =8.314 /(mol-K)) and the system operates between T, = 800 K and T, = 300 K. In the 1st step (ab), the volume changes from V =0.001 m2 to V = 0.005 m. The heat input in this step is V. Q=S*Tas = RT, In 4-1 (0) (2) In the adiabatic processes in the 2nd and 4th steps, PV' = constant (3) is obeyed where y = 5/3 for an ideal gas. Write a script file HW7_1_YourLastName.m that (1) draws the P-V diagram as the figure above (shading is not needed). As a unit of P, use bar (do not use Pascal). To draw the graph, calculate Vand V, from the ideal gas law and Eq. (3). Then, P can be defined as a function of V for each step. (2) calculates the work done by the engine -W = PAV + SPAV +SPOV +SPDV. (4) 3) In evaluating Eq. (4), use MATLAB built-in function "integral". Then, from Eqs. (2) and (4), calculate the engine efficiency defined as -W n= Q (5) 5 T: Show that the calculated efficiency is the same as the Carnot efficiency n=1- T
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


