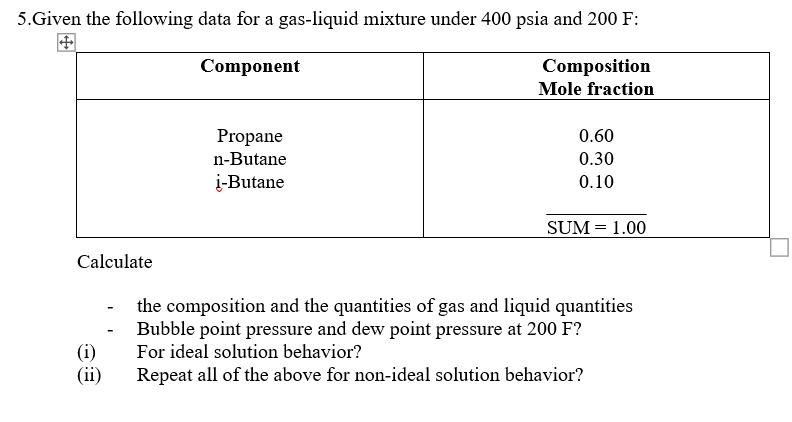
Question: please don't be handwritten 5. Given the following data for a gas-liquid mixture under 400 psia and 200 F: Component Composition Mole fraction Propane n-Butane
please don't be handwritten
5. Given the following data for a gas-liquid mixture under 400 psia and 200 F: Component Composition Mole fraction Propane n-Butane i-Butane 0.60 0.30 0.10 SUM = 1.00 Calculate the composition and the quantities of gas and liquid quantities Bubble point pressure and dew point pressure at 200 F? For ideal solution behavior? Repeat all of the above for non-ideal solution behavior? (ii) 5. Given the following data for a gas-liquid mixture under 400 psia and 200 F: Component Composition Mole fraction Propane n-Butane i-Butane 0.60 0.30 0.10 SUM = 1.00 Calculate the composition and the quantities of gas and liquid quantities Bubble point pressure and dew point pressure at 200 F? For ideal solution behavior? Repeat all of the above for non-ideal solution behavior? (ii)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


