Question: please dont copy others answer. The solved answer is wrong 3) A two-stage parallel feed evaporator is used to concentrate a sugar (sucrose) solution from
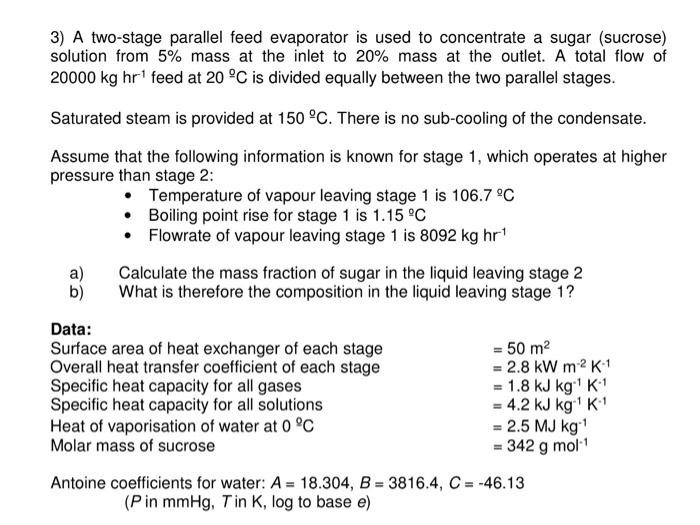
3) A two-stage parallel feed evaporator is used to concentrate a sugar (sucrose) solution from 5% mass at the inlet to 20% mass at the outlet. A total flow of 20000kghr1 feed at 20C is divided equally between the two parallel stages. Saturated steam is provided at 150C. There is no sub-cooling of the condensate. Assume that the following information is known for stage 1, which operates at higher pressure than stage 2 : - Temperature of vapour leaving stage 1 is 106.7C - Boiling point rise for stage 1 is 1.15C - Flowrate of vapour leaving stage 1 is 8092kghr1 a) Calculate the mass fraction of sugar in the liquid leaving stage 2 b) What is therefore the composition in the liquid leaving stage 1? Data: Surface area of heat exchanger of each stage =50m2 Overall heat transfer coefficient of each stage =2.8kWmm2K1 Specific heat capacity for all gases =1.8kJkg1K1 Specific heat capacity for all solutions =4.2kJkg1K1 Heat of vaporisation of water at 0C =2.5MJkg1 Molar mass of sucrose Antoine coefficients for water: A=18.304,B=3816.4,C=46.13 ( P in mmHg,T in K,log to base e )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


