Question: please dont use excel for part b) hand written only. Question Four Sorbitol is a sugar alcohol naturally found in fruits that is often used
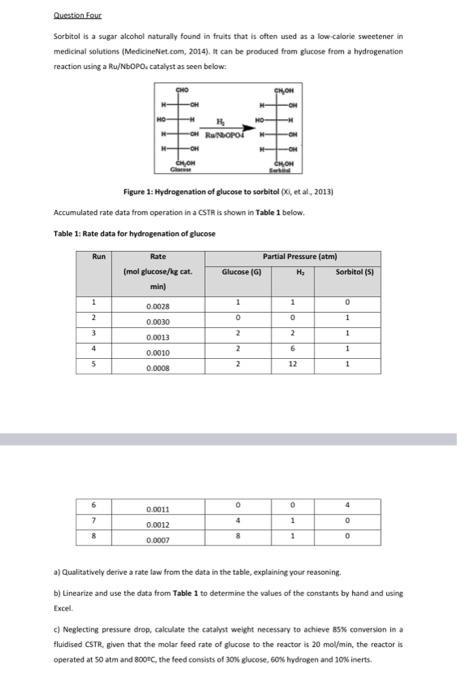
Question Four Sorbitol is a sugar alcohol naturally found in fruits that is often used as a low calorie sweetener in medicinal solutions (MedicineNet.com, 2014). It can be produced from glucose from a hydrogenation reaction using a Ru/NbOPO. catalyst as seen below: CHOM HO HROPOF WOW Figure 1: Hydrogenation of glucose to sorbitol (X, et al. 2013) Accumulated rate data from operation in a CST is shown in Table 1 below Table 1: Rate data for hydrogenation of glucose Rate (mol glucose/x cat min) Run Partial Pressure (atm) Glucose (6) . Sorbitol (51 1 1 1 0 2 0 0 1 0.0028 0.0030 0.0013 3 2 2 1 4 2 6 1 5 0.0010 0.0008 2 12 1 0 0 4 0.0011 6 7 8 4 0 0.0012 0.0007 1 1 8 0 a) Qualitatively derive a rate law from the data in the table, explaining your reasoning b) Linearize and use the data from Table 1 to determine the values of the constants by hand and using Excel c) Neglecting pressure drop, calculate the catalyst weight necessary to achieve 85% conversion in a fludised CSTR. given that the molar feed rate of glucose to the reactor is 20 mol/min, the reactor is operated at 50 atm and 80oC, the feed comists of 30% glucose, som hydrogen and 10% inerts
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


