Question: please double check first answer and solve second. thank you. ochem / biochem introduction 6. (3 points) Why is cyclohexane's experimental result so different than
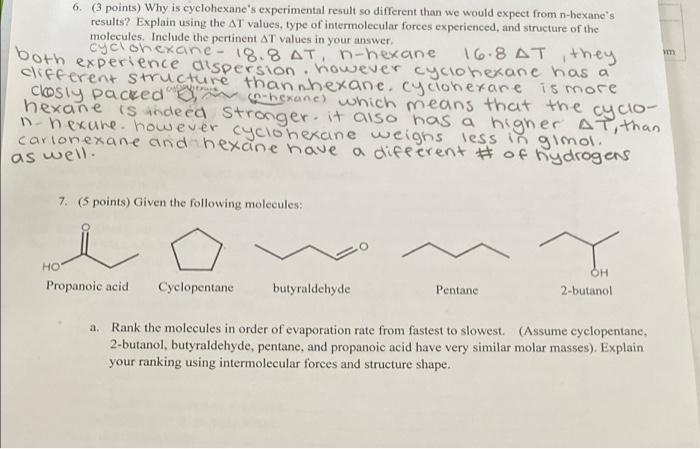
6. (3 points) Why is cyclohexane's experimental result so different than we would expect from n-hexane's results? Explain using the AT values, type of intermolecular forces experienced, and structure of the molecules. Include the pertinent AT values in your answer. 18.8 AT 16.8 AT they both experience dispersion. however cyclohexane has a clifferent structure than nhexane, cyclonerare is more closiy packed om hexanes which means that the cyclo hexane is indeed stronger, it also has a higher AT, than n-nexure. however cyclohexcine weighs less in gimol. carionexane and hexane have a different # of hydrogens as well. 7. (5 points) Given the following molecules: OH HO Propanoic acid Cyclopentane butyraldehyde Pentane 2-butanol a. Rank the molecules in order of evaporation rate from fastest to slowest (Assume cyclopentane, 2-butanol, butyraldehyde, pentane, and propanoic acid have very similar molar masses). Explain your ranking using intermolecular forces and structure shape
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


