Question: Please draw a flow diagram, please use single pass conversion. Please explain every step. Answers are provided. I've been struggling and need a thorough explaination.
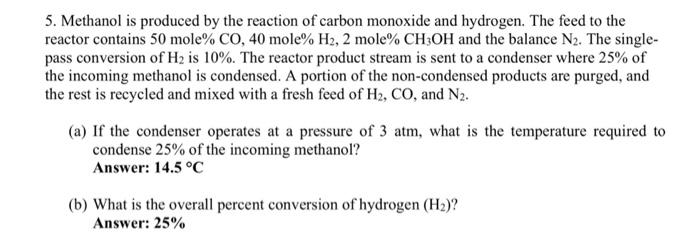
5. Methanol is produced by the reaction of carbon monoxide and hydrogen. The feed to the reactor contains 50 mole %CO,40 mole %H2,2 mole %CH3OH and the balance N2. The singlepass conversion of H2 is 10%. The reactor product stream is sent to a condenser where 25% of the incoming methanol is condensed. A portion of the non-condensed products are purged, and the rest is recycled and mixed with a fresh feed of H2,CO, and N2. (a) If the condenser operates at a pressure of 3atm, what is the temperature required to condense 25% of the incoming methanol? Answer: 14.5C (b) What is the overall percent conversion of hydrogen (H2) ? Answer: 25\%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


