Question: Please draw a full electron pushing mechanism with intermediates please. CI NH .HCI H2N NH2 NH2 CI H3C0 KOH, EtOH, H2O H3CO a. Re-Draw the
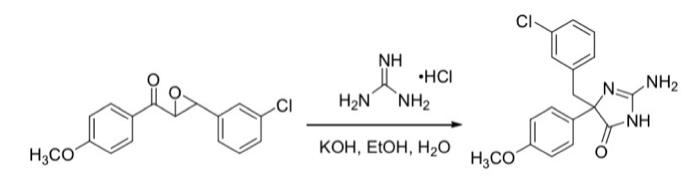

CI NH .HCI H2N NH2 NH2 CI H3C0 KOH, EtOH, H2O H3CO a. Re-Draw the full electron-pushing mechanism for this synthetic reaction. Use the provided literature and/or your Chem 210/212 mechanism knowledge to help you with the mechanism. Note that all elementary steps, including proton transfers, must be explicitly drawn in your mechanism (even if they aren't depicted fully in the literature). For this reaction, you do NOT have to show how the epoxide forms the dicarbonyl intermediate, just that it does. Draw the mechanistic pathway where the ring is formed first and the re-arrangement happens after guanidine addition into one of the carbonyls. (4 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


