Question: please draw an accurate and correct flowchart graph like in the second picture using information provided in the first one. the two crude compounds used
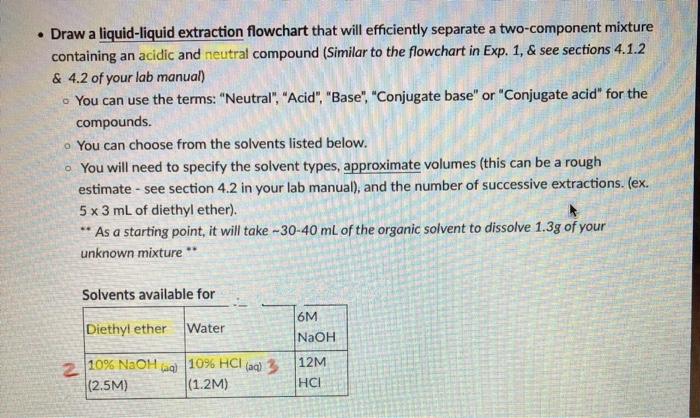

- Draw a liquid-liquid extraction flowchart that will efficiently separate a two-component mixture containing an acidic and neutral compound (Similar to the flowchart in Exp. 1, \& see sections 4.1.2 \& 4.2 of your lab manual) You can use the terms: "Neutral", "Acid", "Base", "Conjugate base" or "Conjugate acid" for the compounds. You can choose from the solvents listed below. You will need to specify the solvent types, approximate volumes (this can be a rough estimate - see section 4.2 in your lab manual), and the number of successive extractions. (ex. 53mL of diethyl ether). "As a starting point, it will take 3040mL of the organic solvent to dissolve 1.3g of your unknown mixture Part A: Liquid-Liquid Extraction Neutral Compound + Acidic Compound White residue = ( 1.4 g of unknown) loss of compound Must have 0.450.75q of both compounds Maximum 40mL of organic solvent
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


