Question: Please edit my lab report to meet all requirements :) thank you Section 1: In this lab investigation, the heat change associated with the dissolution


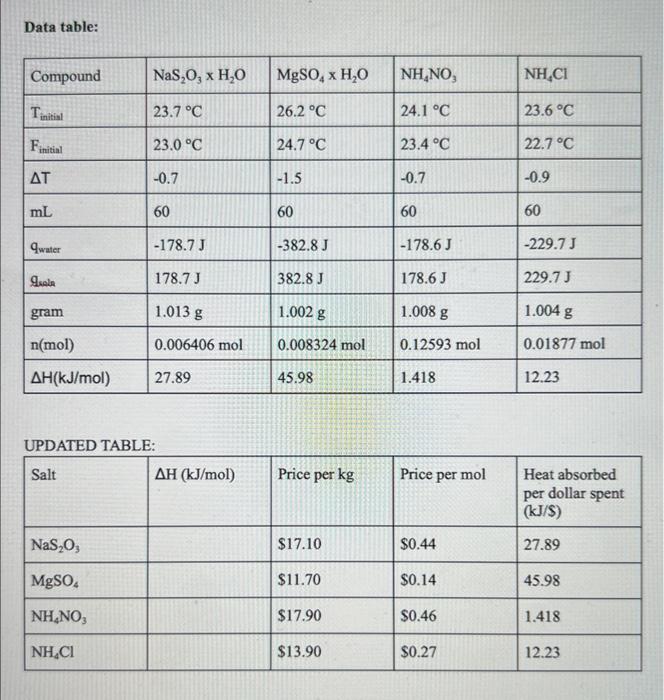

Section 1: In this lab investigation, the heat change associated with the dissolution of each salts being used was determined. With the guiding question of "which salt and in what quantity should be used to make an effective but economical cold pack?" A cold pack could be used for various things, one important factor is such as injury treatment. It is important to know how to implement knowledge from this experiment into real life situations. The dissolution of an ionic compound mixed with water determines the enthalpy change. In this investigation, four different types of salts were used to help determine the changes in enthalpy in order to determine which one is most effective for creating a cold pack. The system gains energy when the salt dissolves in the water due to the energy breaking the attraction between the forces that hold the molecules of the water together in the solvent. The system loses energy when the energy is being released because it causes the attractive forces to form again. Hsoln could be exothermic or endothermic, it varies on how the energy is changing in the system. This can be determined|by looking at the Hsoln during the two processes. Exothermic is when a chemical reaction or physical change releases heat/energy. Endothermic is the process of a chemical reaction or physical change that absorbs heat/energy. Dissolution is the process that a solute in a solid, liquid, or gas phase dissolves in solvent in order to form a solution. Enthalpy is the thermodynamics of chemical and physical processes. Enthalpy can be used to answer the guiding question because one can determine the Hsoln of each salt by mixing it with water in a calorimeter. The four types of salts used in this experiment: 1. NaS2O3 : sodium thiosulfate 2. MgSO4 : magnesium sulfate 3. NH4NO3 : ammonium nitrate 4. NH4Cl : ammonium chloride Section 2: The methods used to obtain the enthalpy of solution were to calculate the molar heat of solution using the data collected during the experiment. First step is to calculate the energy absorbed or heat was absorbed or released per mole ( Hsoln). The coffee cup is meant to protect the reaction that is inside of the cup from heat being released and reduce it from escaping as much as possible, since this experiment calls for the calculations of the heat transferred. Error was reduced in this experiment by adding a lid in order to protect the sides of the calorimeter from releasing any heat to the surroundings. If 100mL of water was used as opposed to 60mL, the enthalpy of dissolution for each salt result would have been different. This is because there will be changes in the temperature due to the enthalpy of solution depending on the volume of the solvent. The larger the volume, the larger the change in temperature. The smaller the volume, the less change in temperature. Calculations: NaS2O3158.11g/mol1.013g=0.006406mol(61.013g)(4.184)(0.7)=q=178.69JH2Hq=178.69Jsol0.71.013=18gx(g)1.013(18)=x(0.7)0.718.013=0.70.7x25.73/1,000=0.0257=$0.44MgSO461.002(4.184)(1.5)=382.84851.002=0.008325mol=45,987.81=45.988kJ/mol120.36661.004(4.184)(0.9)=229.716712238.501=12.239kJ/mol1.51.002=18xx=12.024gx1,0001kg=0.01202x=$0.14 NH4NO360mL of water +1.008 grams (4.184Jg)(0.7) final temperature =63.9368 1.008g=0.01259mol80.04g/mol63.9368=5,07837967Joules/1000=5.078kJ/mol0.012590.71.008=1.4418degreesC=25.92g10001kg=0.0259217.9costofsalt=$0.46NH4Cl53.49g/mol1.004g=18x0.91.004(18)=0.90.9xx=1,00020.08=0.0200813.90=$0.27 Section 3: After observing the cost of each salt, the best salt to make an efficient and economical cold pack is magnesium sulfate. This is because it has the lowest cost of $0.14 per pack at drop temperature of 18C compared to the other salts as shown in the calculations above. The values of the cost per gram per change in temperature contrast because as temperature increases, the enthalpy increases. A difference that was noticed when comparing data with peers was the efficient salt considered. The majority of the groups claimed that NH4Cl was the most efficient, while a few others claimed NH4NO3 was more efficient. This could have been due to inaccuracy in measurements. Data table: IIPN ATFN TARI F- Section 1: Scientific Concept and Guiding Question Did the author provide sufficient and relevant background information related to key scientific concepts? Section 1: Scientific Concept and Guiding Question Is the background information accurate? Section 1: Scientific Concept and Guiding Question Did the author make the guiding question explicit in describing the goal of the investigation? Section 1: Scientific Concept and Guiding Question Did the author explain how the guiding question is related to the key scientific concepts? Section 2: Data Collection and Analysis Did the author correctly describe the procedure with sufficient but not excessive detail? Section 2: Data Collection and Analysis Did the author correctly describe the procedure with a sufficient and appropriate explanation? Section 2: Data Collection and Analysis Did the author explain what data were collected during the investigation and why they were collected? Section 2: Data Collection and Analysis Did the author describe how the data was analyzed and provide sample calculations? Section 2: Data Collection and Analysis Did the author identify sources of error and explain steps taken to reduce error in the investigation? Section 3: Argument Did the author provide a claim that answers the guiding question? Section 3: Argument Did the author use data as evidence in the argument? Section 3: Argument Is the claim consistent with all of the evidence? Section 3: Argument Did the author present the evidence in an appropriate manner by using a correctly formatted and labeled graph, table, or figure? Section 3: Argument Did the author present the evidence in an appropriate manner by using a correct significant figures and units (e,g,m/s,g,mL)? Section 3: Argument Did the author include a justification of the evidence that explains how the evidence supports the claim? Section 3: Argument Did the author include a justification of the evidence that defends the inclusion of the evidence with a specific science concept or by discussing any underlying assumptions? Mechanics Organization: Are there three distinct sections? Are sentences linked together in a logical order? Do paragraphs begin with a topic sentence? Mechanics Grammar: Complete sentences, proper subject-verb agreement, etc. Mechanics Conventions: Correct spelling, punctuation. capitalizations, proper use of subscripts and superscripts or special symbols? Mechanics Style and Tone: Did the author write in third person in a tone that is professional and impartial? Did the author reference the evidence in an appropriate manner (e.g., supports and suggests vs "proves") Section 1: In this lab investigation, the heat change associated with the dissolution of each salts being used was determined. With the guiding question of "which salt and in what quantity should be used to make an effective but economical cold pack?" A cold pack could be used for various things, one important factor is such as injury treatment. It is important to know how to implement knowledge from this experiment into real life situations. The dissolution of an ionic compound mixed with water determines the enthalpy change. In this investigation, four different types of salts were used to help determine the changes in enthalpy in order to determine which one is most effective for creating a cold pack. The system gains energy when the salt dissolves in the water due to the energy breaking the attraction between the forces that hold the molecules of the water together in the solvent. The system loses energy when the energy is being released because it causes the attractive forces to form again. Hsoln could be exothermic or endothermic, it varies on how the energy is changing in the system. This can be determined|by looking at the Hsoln during the two processes. Exothermic is when a chemical reaction or physical change releases heat/energy. Endothermic is the process of a chemical reaction or physical change that absorbs heat/energy. Dissolution is the process that a solute in a solid, liquid, or gas phase dissolves in solvent in order to form a solution. Enthalpy is the thermodynamics of chemical and physical processes. Enthalpy can be used to answer the guiding question because one can determine the Hsoln of each salt by mixing it with water in a calorimeter. The four types of salts used in this experiment: 1. NaS2O3 : sodium thiosulfate 2. MgSO4 : magnesium sulfate 3. NH4NO3 : ammonium nitrate 4. NH4Cl : ammonium chloride Section 2: The methods used to obtain the enthalpy of solution were to calculate the molar heat of solution using the data collected during the experiment. First step is to calculate the energy absorbed or heat was absorbed or released per mole ( Hsoln). The coffee cup is meant to protect the reaction that is inside of the cup from heat being released and reduce it from escaping as much as possible, since this experiment calls for the calculations of the heat transferred. Error was reduced in this experiment by adding a lid in order to protect the sides of the calorimeter from releasing any heat to the surroundings. If 100mL of water was used as opposed to 60mL, the enthalpy of dissolution for each salt result would have been different. This is because there will be changes in the temperature due to the enthalpy of solution depending on the volume of the solvent. The larger the volume, the larger the change in temperature. The smaller the volume, the less change in temperature. Calculations: NaS2O3158.11g/mol1.013g=0.006406mol(61.013g)(4.184)(0.7)=q=178.69JH2Hq=178.69Jsol0.71.013=18gx(g)1.013(18)=x(0.7)0.718.013=0.70.7x25.73/1,000=0.0257=$0.44MgSO461.002(4.184)(1.5)=382.84851.002=0.008325mol=45,987.81=45.988kJ/mol120.36661.004(4.184)(0.9)=229.716712238.501=12.239kJ/mol1.51.002=18xx=12.024gx1,0001kg=0.01202x=$0.14 NH4NO360mL of water +1.008 grams (4.184Jg)(0.7) final temperature =63.9368 1.008g=0.01259mol80.04g/mol63.9368=5,07837967Joules/1000=5.078kJ/mol0.012590.71.008=1.4418degreesC=25.92g10001kg=0.0259217.9costofsalt=$0.46NH4Cl53.49g/mol1.004g=18x0.91.004(18)=0.90.9xx=1,00020.08=0.0200813.90=$0.27 Section 3: After observing the cost of each salt, the best salt to make an efficient and economical cold pack is magnesium sulfate. This is because it has the lowest cost of $0.14 per pack at drop temperature of 18C compared to the other salts as shown in the calculations above. The values of the cost per gram per change in temperature contrast because as temperature increases, the enthalpy increases. A difference that was noticed when comparing data with peers was the efficient salt considered. The majority of the groups claimed that NH4Cl was the most efficient, while a few others claimed NH4NO3 was more efficient. This could have been due to inaccuracy in measurements. Data table: IIPN ATFN TARI F- Section 1: Scientific Concept and Guiding Question Did the author provide sufficient and relevant background information related to key scientific concepts? Section 1: Scientific Concept and Guiding Question Is the background information accurate? Section 1: Scientific Concept and Guiding Question Did the author make the guiding question explicit in describing the goal of the investigation? Section 1: Scientific Concept and Guiding Question Did the author explain how the guiding question is related to the key scientific concepts? Section 2: Data Collection and Analysis Did the author correctly describe the procedure with sufficient but not excessive detail? Section 2: Data Collection and Analysis Did the author correctly describe the procedure with a sufficient and appropriate explanation? Section 2: Data Collection and Analysis Did the author explain what data were collected during the investigation and why they were collected? Section 2: Data Collection and Analysis Did the author describe how the data was analyzed and provide sample calculations? Section 2: Data Collection and Analysis Did the author identify sources of error and explain steps taken to reduce error in the investigation? Section 3: Argument Did the author provide a claim that answers the guiding question? Section 3: Argument Did the author use data as evidence in the argument? Section 3: Argument Is the claim consistent with all of the evidence? Section 3: Argument Did the author present the evidence in an appropriate manner by using a correctly formatted and labeled graph, table, or figure? Section 3: Argument Did the author present the evidence in an appropriate manner by using a correct significant figures and units (e,g,m/s,g,mL)? Section 3: Argument Did the author include a justification of the evidence that explains how the evidence supports the claim? Section 3: Argument Did the author include a justification of the evidence that defends the inclusion of the evidence with a specific science concept or by discussing any underlying assumptions? Mechanics Organization: Are there three distinct sections? Are sentences linked together in a logical order? Do paragraphs begin with a topic sentence? Mechanics Grammar: Complete sentences, proper subject-verb agreement, etc. Mechanics Conventions: Correct spelling, punctuation. capitalizations, proper use of subscripts and superscripts or special symbols? Mechanics Style and Tone: Did the author write in third person in a tone that is professional and impartial? Did the author reference the evidence in an appropriate manner (e.g., supports and suggests vs "proves")
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


