Question: Please either text or computer generate your response as some hand written answers are a bit hard to read! and provide brief example in simple
Please either text or computer generate your response as some hand written answers are a bit hard to read! and provide brief example in simple terms
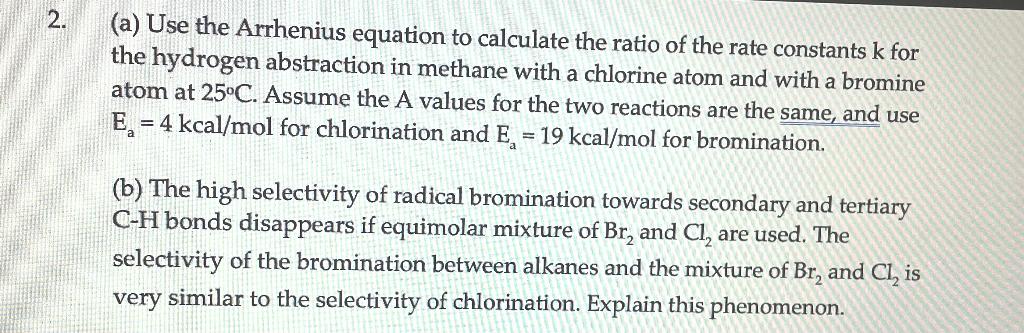
(a) Use the Arrhenius equation to calculate the ratio of the rate constants k for the hydrogen abstraction in methane with a chlorine atom and with a bromine atom at 25C. Assume the A values for the two reactions are the same, and use Ea=4kcal/mol for chlorination and Ea=19kcal/mol for bromination. (b) The high selectivity of radical bromination towards secondary and tertiary C-H bonds disappears if equimolar mixture of Br2 and Cl2 are used. The selectivity of the bromination between alkanes and the mixture of Br2 and Cl2 is very similar to the selectivity of chlorination. Explain this phenomenon
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


