Question: please explain 3ii. i dont understand it at all. please break it down in simple terms. 3. Consider compound A. double bonds are -Spa SP
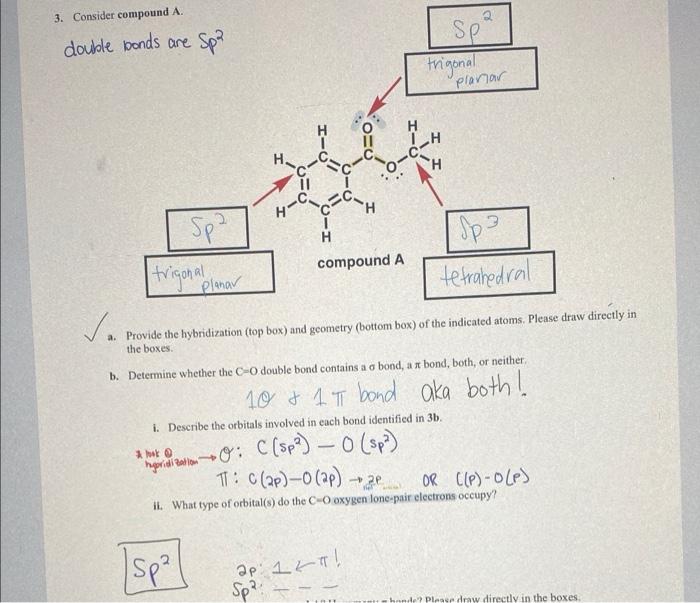
3. Consider compound A. double bonds are -Spa SP trigonal planar H CH CH H C1 11 H-C C Spa compound A I Spa tetrahedral trigonal Planar va a. Provide the hybridization (top box) and geometry (bottom box) of the indicated atoms. Please draw directly in the boxes b. Determine whether the CO double bond contains a o bond, a x bond, both, or neither i. Describe the orbitals involved in each bond identified in 3b. - 10 & 1 T bond aka both! on the comm0: C (Spa) 0 (sp) TT: C(2P) - (ap) OR ((P)-OLP) - 2 il. What type of orbital(s) do the Cooxygen lonc pair electrons occupy? Isp?] ap 1 ka! Sp?! hande? Please draw directly in the boxes
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


