Question: Please explain a little bit while your doing it so i can understand this type of exercise Thank you! A factory produces hot cooling water
Please explain a little bit while your doing it so i can understand this type of exercise Thank you!
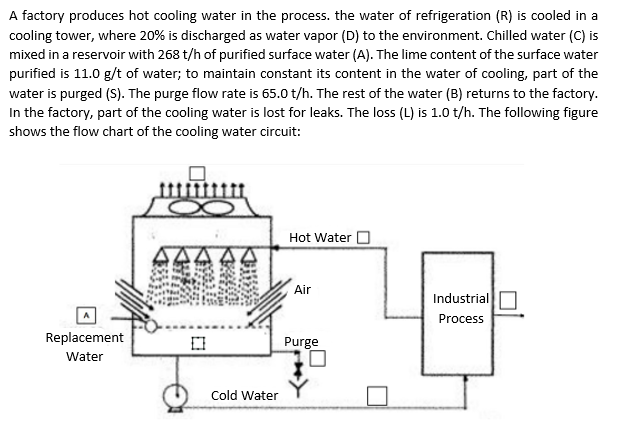
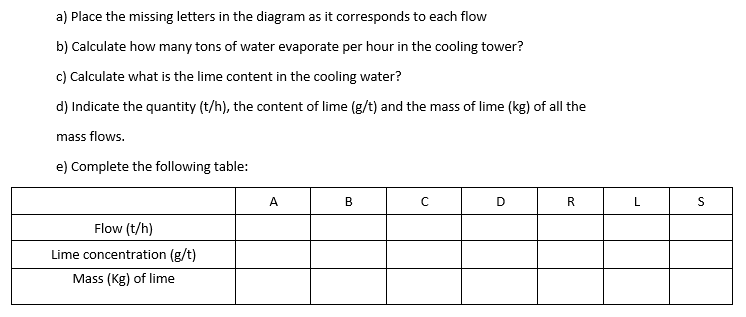
A factory produces hot cooling water in the process. the water of refrigeration (R) is cooled in a cooling tower, where 20% is discharged as water vapor (D) to the environment. Chilled water (C) is mixed in a reservoir with 268 t/h of purified surface water (A). The lime content of the surface water purified is 11.0 g/t of water; to maintain constant its content in the water of cooling, part of the water is purged (S). The purge flow rate is 65.0 t/h. The rest of the water (B) returns to the factory. In the factory, part of the cooling water is lost for leaks. The loss (L) is 1.0 t/h. The following figure shows the flow chart of the cooling water circuit: Hot Water Air Industrial Process Replacement Water Purge Cold Water a) Place the missing letters in the diagram as it corresponds to each flow b) Calculate how many tons of water evaporate per hour in the cooling tower? c) Calculate what is the lime content in the cooling water? d) Indicate the quantity (t/h), the content of lime (g/t) and the mass of lime (kg) of all the mass flows. e) Complete the following table: A B D R L S Flow (t/h) Lime concentration (g/t) Mass (Kg) of lime
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


