Question: Please explain and show all work Given the following equation: 4 NH3 (s) + 5O2 (g) 4 NO (8) + 6 H20 (1) What is
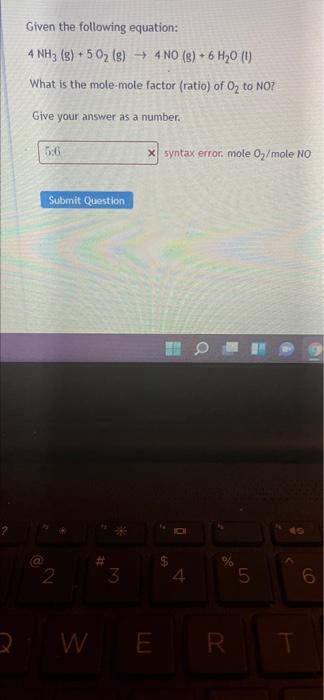
Given the following equation: 4 NH3 (s) + 5O2 (g) 4 NO (8) + 6 H20 (1) What is the mole-mole factor (ratio) of O2 to NO? Give your answer as a number. 536 x syntax error mole 07/mole NO Submit Question NO 3 4. 15 6 09 3 W E R W E
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


