Question: please explain by writing this out 12) (5 points) Find the heat required (in kJ ) to heat 41.0g of 120 from 7.00oC to 125C
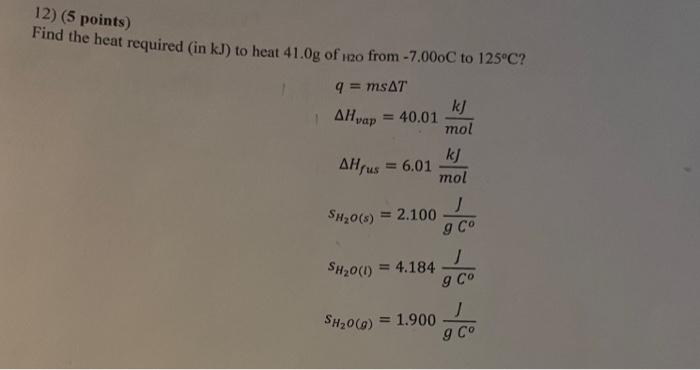
12) (5 points) Find the heat required (in kJ ) to heat 41.0g of 120 from 7.00oC to 125C ? q=msTHvap=40.01molkJHfus=6.01molkJsH2O(s)=2.100gCJsH2O(l)=4.184gCJsH2O(g)=1.900gCJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


