Question: For the hydrogen atom, the relative probability of finding an electron at any single point in an orbital n,(0, , r) is 4 (0,4,r).
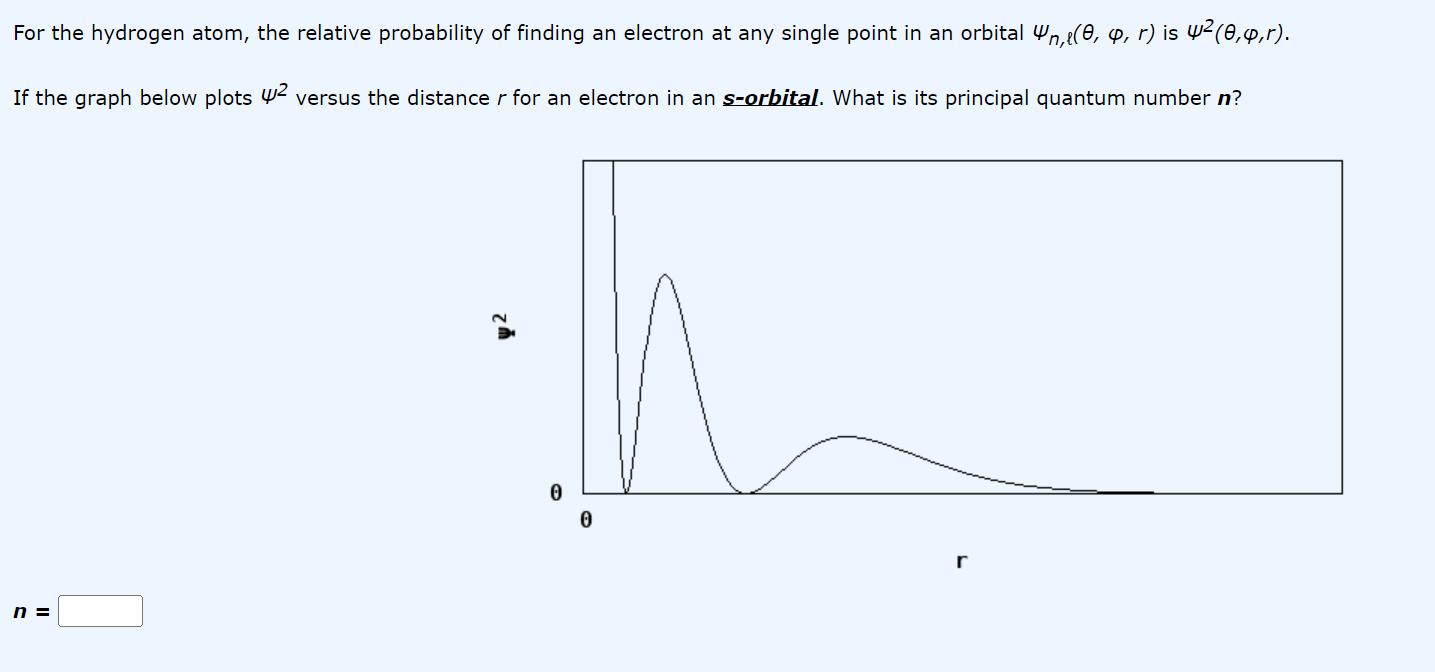
For the hydrogen atom, the relative probability of finding an electron at any single point in an orbital n,(0, , r) is 4 (0,4,r). If the graph below plots 2 versus the distance r for an electron in an s-orbital. What is its principal quantum number n? n = 0 r
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The graph you provided suggests that the relative probability of finding an electron at any distance ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


