Question: please explain each atep in GREAT detail Question Two The following data for the hydrogenation of i-octene to form i-octane were obtained using a differential
please explain each atep in GREAT detail 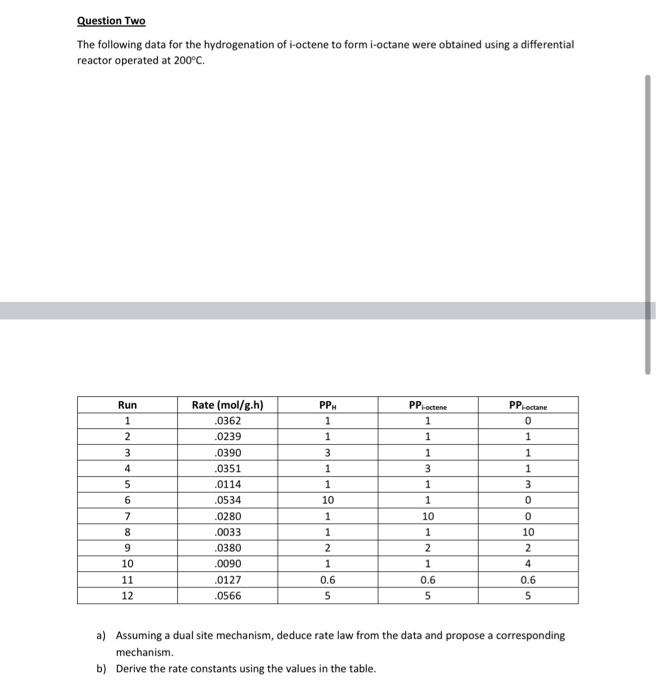

Question Two The following data for the hydrogenation of i-octene to form i-octane were obtained using a differential reactor operated at 200C. PP octene PP-octane Run 1 1 2 3 4 Rate (mol/g.h) .0362 .0239 .0390 .0351 .0114 .0534 .0280 .0033 .0380 .0090 .0127 .0566 PP 1 1 3 1 1 10 1 1 2 1 0.6 5 1 1 3 1 1 5 6 7 8 9 10 11 12 0 1 1 1 3 0 0 10 2 4 0.6 5 10 1 2 1 0.6 5 a) Assuming a dual site mechanism, deduce rate law from the data and propose a corresponding mechanism b) Derive the rate constants using the values in the table
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


