Question: Please explain each part. I don't understand why we need to include the first deltaH (-1225.6kJ) since the reaction only includes P4O10 + 6PCl5--> 10
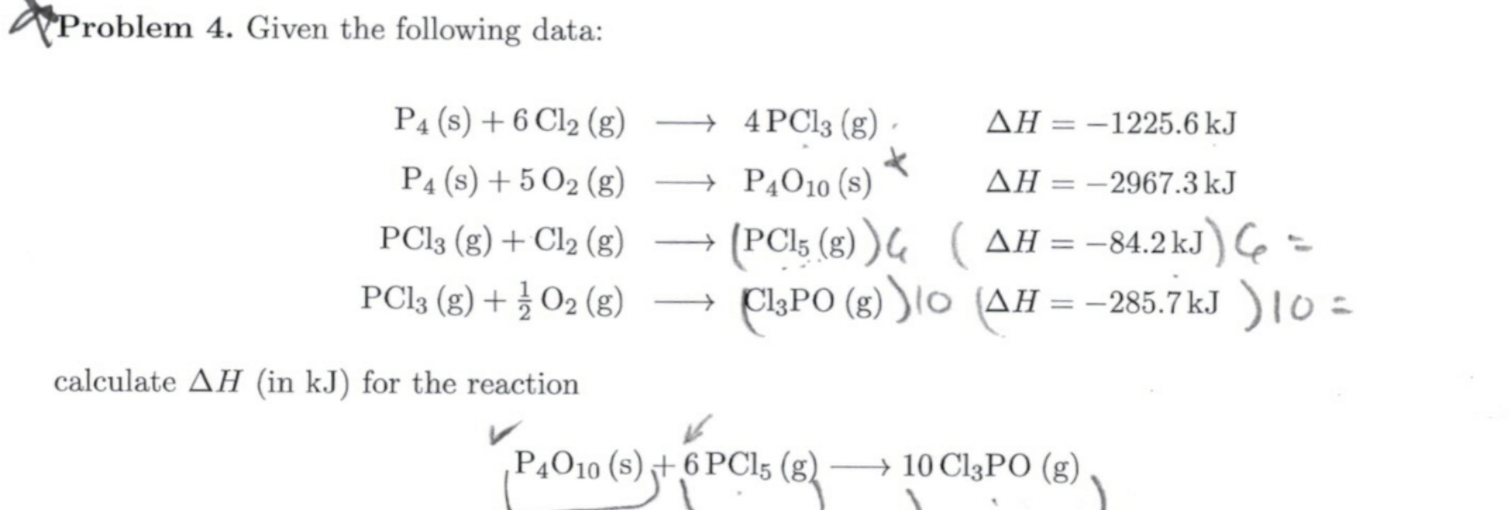 Please explain each part. I don't understand why we need to include the first deltaH (-1225.6kJ) since the reaction only includes P4O10 + 6PCl5--> 10 ClPO????
Please explain each part. I don't understand why we need to include the first deltaH (-1225.6kJ) since the reaction only includes P4O10 + 6PCl5--> 10 ClPO????
Problem 4. Given the following data: AH = -1225.6 kJ * AH = -2967.3 kJ P4 (8) + 6 C12 (8) P4 (s) + 5 O2 (g) PC13 (g) + Cl2 (g) PC13 (g) + O2(g) - 4 PC13 (8) + P4010 (8) + G (PCL5 (g)) ( AH = -84.2 kJ) Ce =) CISPO (g) )/0 (AH = -285.7kJ ) 10 = calculate AH (in kJ) for the reaction P4010 (s) + 6 PC15 (8) ) 6 () 640C18) + 10 C13PO (g)
Step by Step Solution
There are 3 Steps involved in it
To solve this problem we must apply Hesss Law which states If a reaction is carried out in a series of steps the total enthalpy change is the sum of t... View full answer

Get step-by-step solutions from verified subject matter experts


