Question: Please explain each step of this procedure, its a difficult topic for me and I need to understand it very well PS: the exercise is
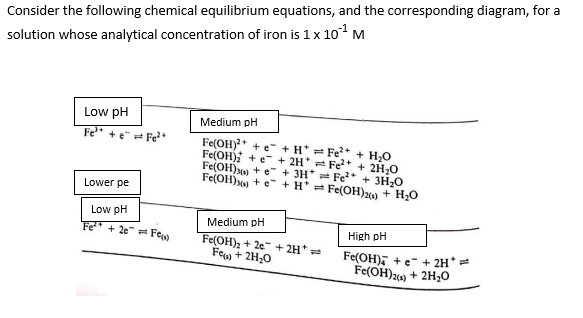
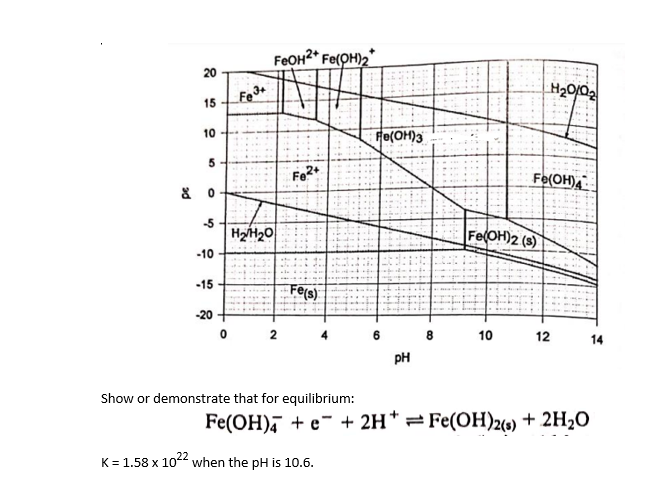
Please explain each step of this procedure, its a difficult topic for me and I need to understand it very well
PS: the exercise is about proving that with those conditions k=1,58x10^22
Consider the following chemical equilibrium equations, and the corresponding diagram, for a solution whose analytical concentration of iron is 1101M Show or demonstrate that for equilibrium: Fe(OH)4+e+2H+Fe(OH)2(s)+2H2O K=1.581022 when the pH is 10.6. Consider the following chemical equilibrium equations, and the corresponding diagram, for a solution whose analytical concentration of iron is 1101M Show or demonstrate that for equilibrium: Fe(OH)4+e+2H+Fe(OH)2(s)+2H2O K=1.581022 when the pH is 10.6
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


