Question: please explain every calculation done during this problem. 1. The elementary gas-phase reaction (CH3)3COOC(CH3)3C2H6+2CH3COCH3AB+2C is carried out isothermally at 400K in a flow reactor with
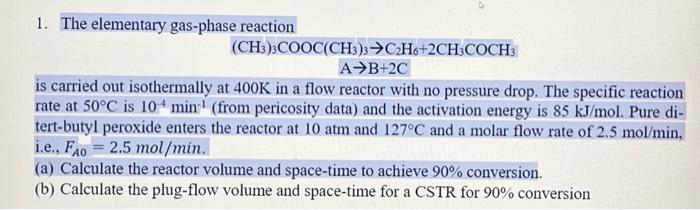
1. The elementary gas-phase reaction (CH3)3COOC(CH3)3C2H6+2CH3COCH3AB+2C is carried out isothermally at 400K in a flow reactor with no pressure drop. The specific reaction rate at 50C is 104min1 (from pericosity data) and the activation energy is 85kJ/mol. Pure ditert-butyl peroxide enters the reactor at 10atm and 127C and a molar flow rate of 2.5mol/min, i.e., FA0=2.5mol/min. (a) Calculate the reactor volume and space-time to achieve 90% conversion. (b) Calculate the plug-flow volume and space-time for a CSTR for 90% conversion
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


