Question: Please explain how part 2 is completed. I attached the necessary information 2) Use the Lambert-Beer law to determine the concentration of methyl orange in

Please explain how part 2 is completed. I attached the necessary information
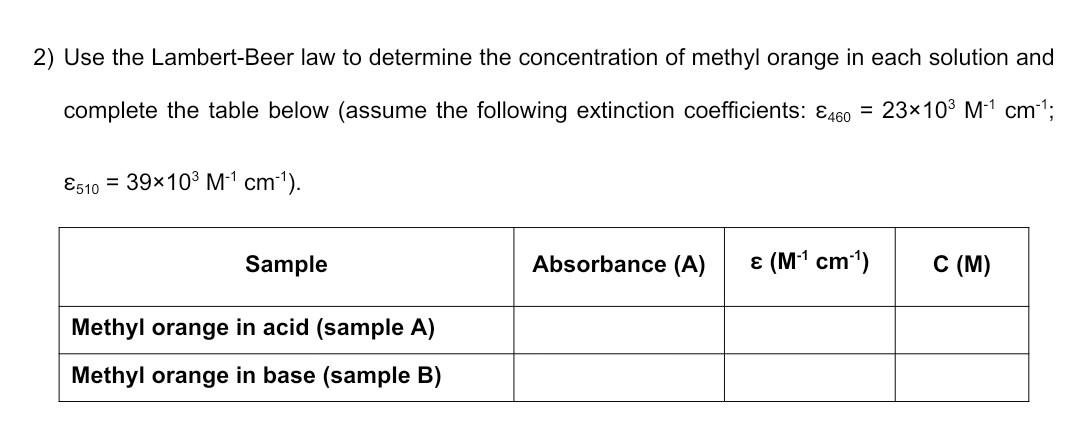
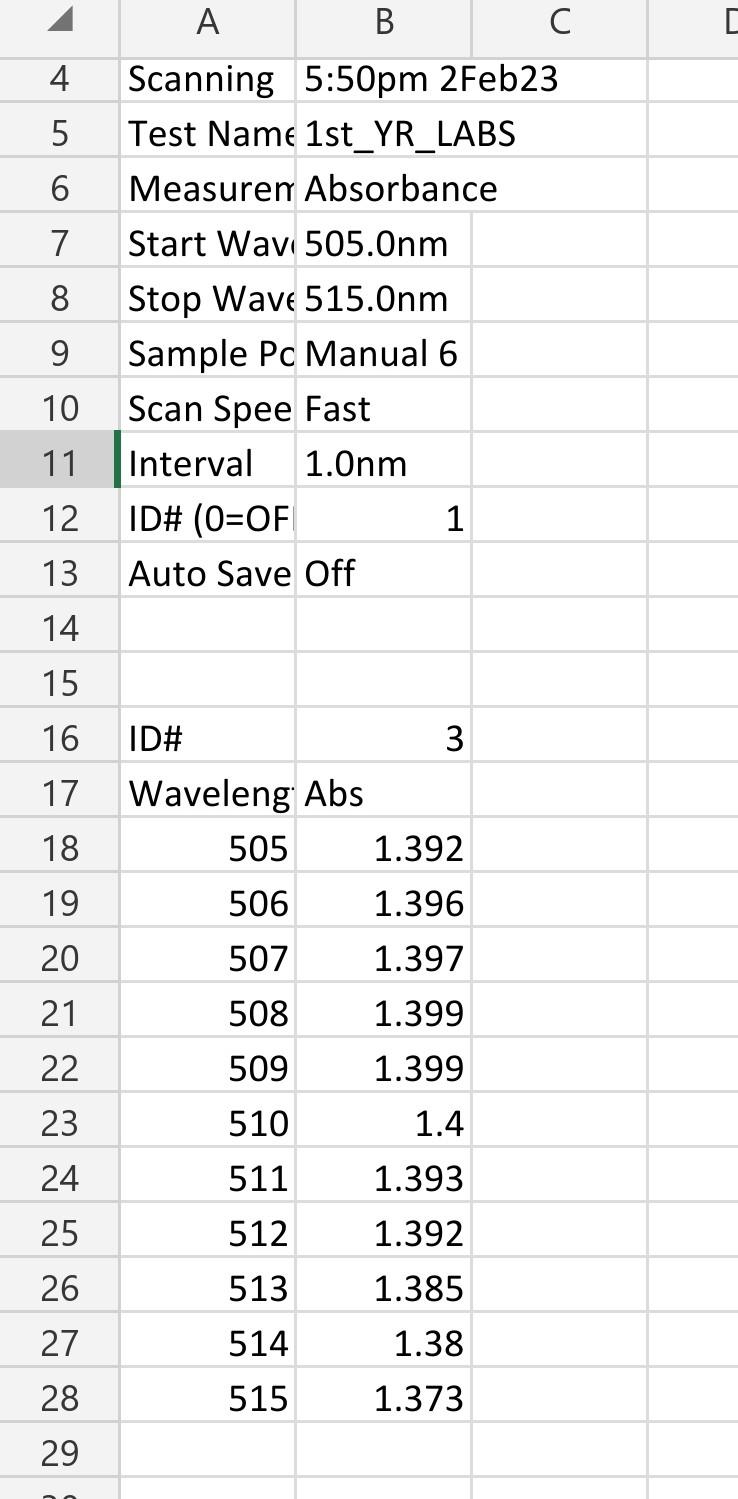
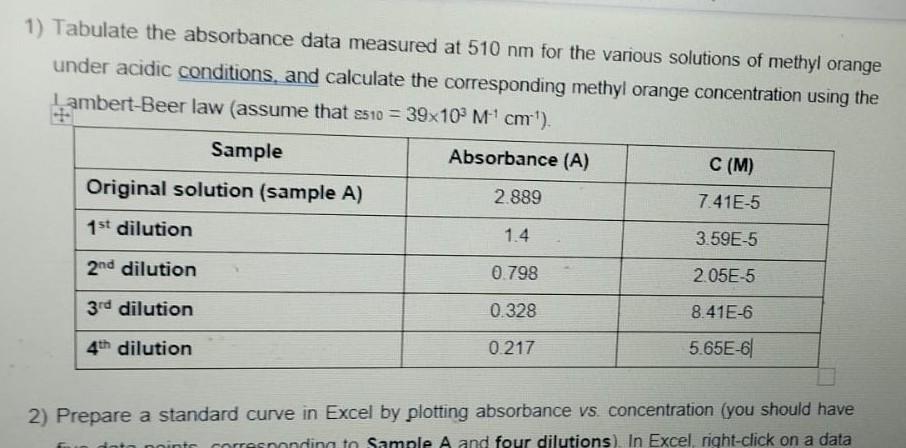
2) Use the Lambert-Beer law to determine the concentration of methyl orange in each solution and complete the table below (assume the following extinction coefficients: 460=23103M1cm1; 510=39103M1cm1). A C Scanning 5:50pm 2Feb23 Test Nam1st_YR_LABS Measuren Absorbance Start Wav 505.0nm Stop Wave 515.0nm Sample Pc Manual 6 Scan Spee Fast Interval 1.0nm ID\# (0=OF 1 Auto Save Off ID\# 3 Waveleng Abs \begin{tabular}{|r|r|} \hline 505 & 1.392 \\ \hline 506 & 1.396 \\ \hline 507 & 1.397 \\ \hline 508 & 1.399 \\ \hline 509 & 1.399 \\ \hline 510 & 1.4 \\ \hline 511 & 1.393 \\ \hline 512 & 1.392 \\ \hline 513 & 1.385 \\ \hline 514 & 1.38 \\ \hline 515 & 1.373 \\ \hline \end{tabular} 1) Tabulate the absorbance data measured at 510nm for the various solutions of methyl orange under acidic conditions, and calculate the corresponding methyl orange concentration using the I ambert-Beer law (assume that 510=39103M1cm1 ). 2) Prepare a standard curve in Excel by plotting absorbance vs. concentration (you should have 1. Prepare two separate solutions of methyl orange by diluting 3-4 drops of stock solution (bench reagent) in 10mL of distilled H2O in a beaker (color should be just noticeable by eye). 2. Acidify one solution by addition of 1-2 mL of dilute hydrochloric acid (HCl), mix with a clean glass rod and label it as sample A. Note the color change. 3. Add 1-2 mL of dilute sodium hydroxide (NaOH) to the other solution, mix with a clean glass rod and label it as sample B. Note the color change. 4. Using a clean dropper to carefully transfer 1mL of each solution to a cuvette. 5. Obtain absorption spectra in the 250-700 nm range for each solution. Get help from your Teaching Assistant when you use the spectrometer. A blank spectrum is first recorded using a cuvette containing just distilled H2O. It is only necessary to acquire the blank once. 6. Make sure to rinse carefully the cuvette with distilled H2O between measurements. 7. Note that the absorbance must not be greater than 1.0. If necessary dilute the sample and record the dilution factor you used. 8. Save the spectra to a USB key
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


