Question: please explain how to get answer t Question 5 074 pts Given the balanced chemical equation: Fe(s) + 2 HCl(aq) - FeCl2lag) + H2(8) AHrxn
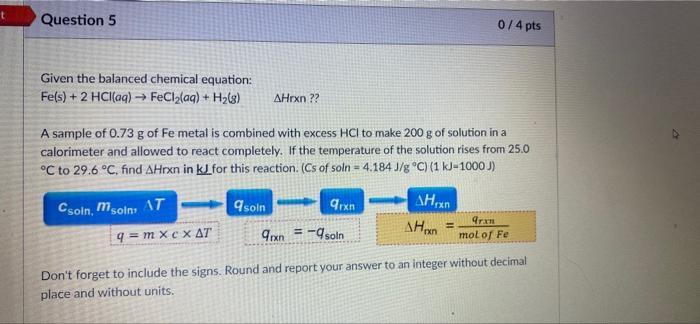
t Question 5 074 pts Given the balanced chemical equation: Fe(s) + 2 HCl(aq) - FeCl2lag) + H2(8) AHrxn ?? A sample of 0.73 g of Fe metal is combined with excess HCl to make 200 g of solution in a calorimeter and allowed to react completely. If the temperature of the solution rises from 25.0 C to 29.6 C, find AHrxn in kJ for this reaction. (Cs of soln = 4.184 J/g C) (1 kJ-1000) soin Arxn Csoin moim, AT 4 = mxc X AT AH AH 9rx = -9 soln Arxn mol of Fe Don't forget to include the signs. Round and report your answer to an integer without decimal place and without units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


