Question: Please explain how to get to the solution 2. An Illinois coal is burned at a rate of 1.00kgs1. If the analysis of the coal
Please explain how to get to the solution
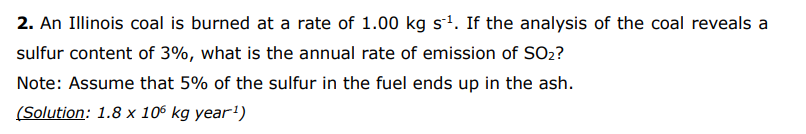
2. An Illinois coal is burned at a rate of 1.00kgs1. If the analysis of the coal reveals a sulfur content of 3%, what is the annual rate of emission of SO2 ? Note: Assume that 5% of the sulfur in the fuel ends up in the ash. (Solution: 1.8106kg year-1) 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


