Question: Please explain how you plot this and how to solve 26.3-2. Comparison of Differential and Flash Distillation. A mixture of 100 kg mol that contains
Please explain how you plot this and how to solve
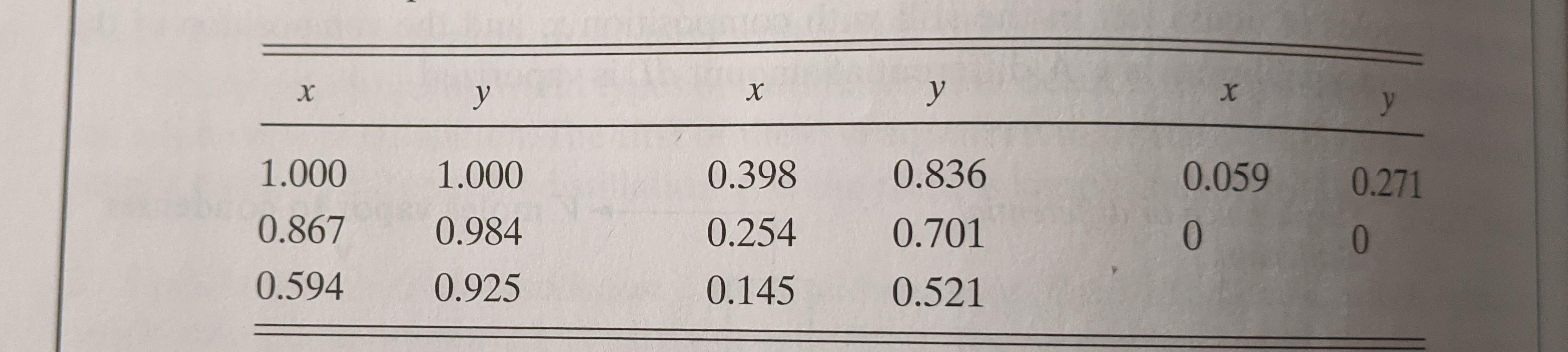
26.3-2. Comparison of Differential and Flash Distillation. A mixture of 100 kg mol that contains 60 mol % n-pentane (A) and 40 mol % n-heptane (B) is vaporized at 101.32 kPa pressure under differential conditions until 40 kg mol are distilled. Use equilibrium data from Example 26.3-2. (a) What is the average composition of the total vapor distilled and the com- position of the remaining liquid? (b) If this same vaporization is done in an equilibrium or flash distillation and 40 kg mol are distilled, what is the composition of the vapor distilled and of the remaining liquid? Ans. (a) x2 = 0.405, Yav = 0.892; (b) x2 = 0.430, y2 = 0.854 1.000 1.000 0.398 0.836 0.059 0.271 0.867 0.984 0.701 0 0 0.254 0.145 0.594 0.925 0.521
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


