Question: Please explain in detail how to solve this problem. The solution posted on Chegg is incomprehensible. Thank you. Updte: Apparently Chegg doesn't allow copy -
Please explain in detail how to solve this problem. The solution posted on Chegg is incomprehensible. Thank you.
Updte: Apparently Chegg doesn't allow copyandpaste! So Im including a screenshot of the question I need help with.At a particular temperature, decomposes according to a firstorder rate law with a
halflife of If the initial concentration of is molecules what will be
the concentration in molecules after
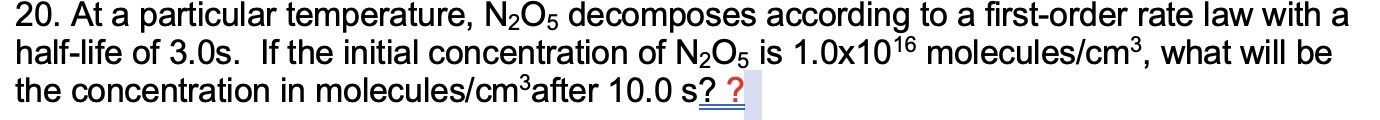
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


