Question: please explain in steps since I'm getting different answers A closed system initially containing 1.000103MH2,2.000103MI2 at 448C is allowed to reach equilibrium. Analysis of the
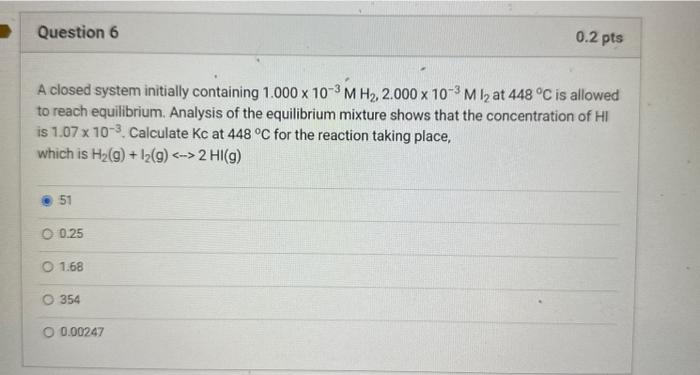
A closed system initially containing 1.000103MH2,2.000103MI2 at 448C is allowed to reach equilibrium. Analysis of the equilibrium mixture shows that the concentration of HI is 1.07103. Calculate Kc at 448C for the reaction taking place, which is H2(g)+I2(g)2HI(g) 51 0.25 1.68 354 0.00247
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


