Question: please explain step by step how to solve the questions 1 and 2 with the correct answer. A gas has a volume of 200. mL
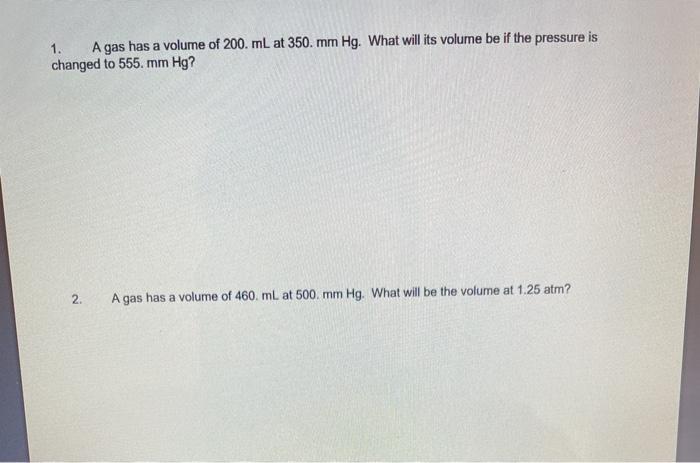
A gas has a volume of 200. mL at 350 mm Hg. What will its volume be if the pressure is changed to 555. mm Hg? 2. A gas has a volume of 460 mL at 500 mm Hg. What will be the volume at 1.25 atm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


