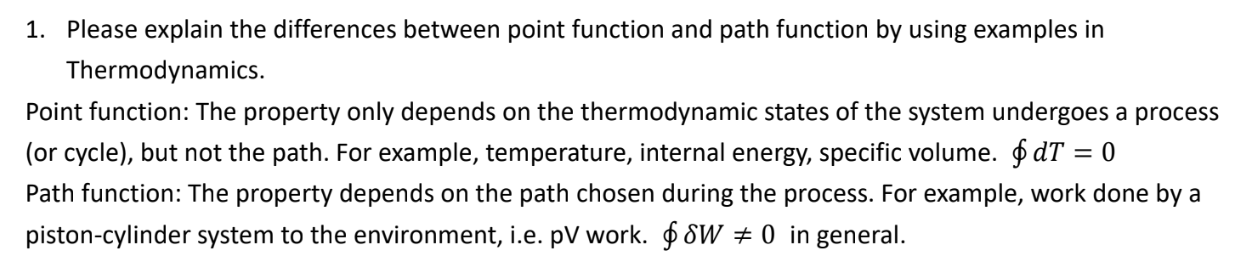
Question: Please explain the differences between point function and path function by using examples in Thermodynamics. Point function: The property only depends on the thermodynamic states
Please explain the differences between point function and path function by using examples in
Thermodynamics.
Point function: The property only depends on the thermodynamic states of the system undergoes a process
or cycle but not the path. For example, temperature, internal energy, specific volume.
Path function: The property depends on the path chosen during the process. For example, work done by a
pistoncylinder system to the environment, ie pV work. in general.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


