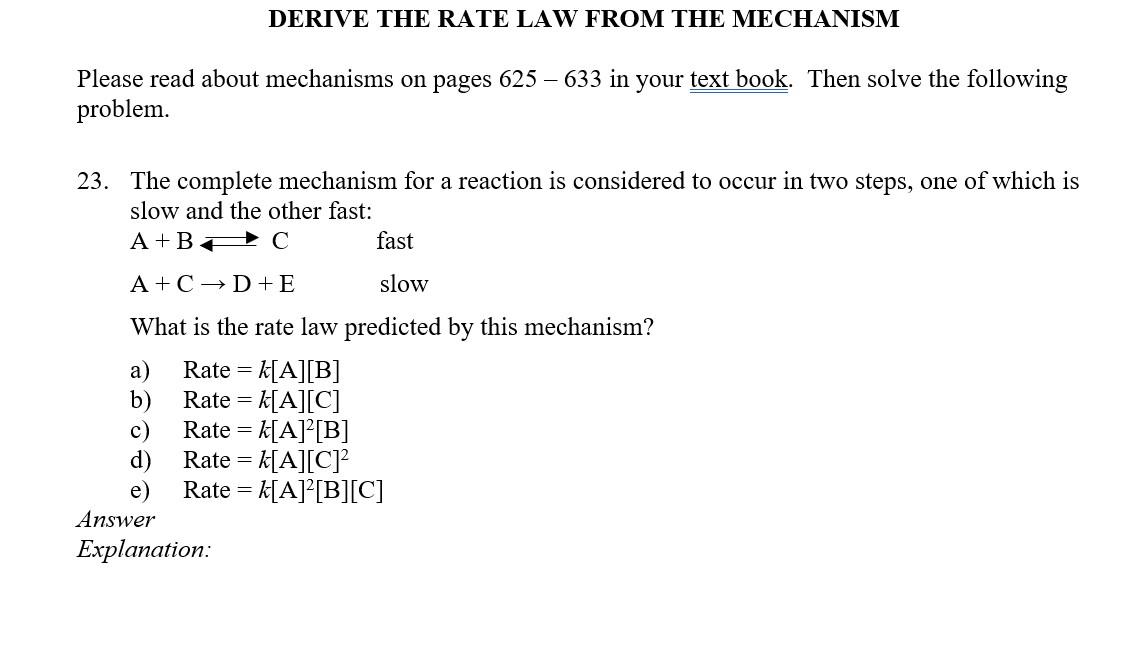
Question: Please explain the slow step because it still confuse me Please read about mechanisms on pages 625633 in your text book. Then solve the following
 Please explain the slow step because it still confuse me
Please explain the slow step because it still confuse me
Please read about mechanisms on pages 625633 in your text book. Then solve the following problem. 23. The complete mechanism for a reaction is considered to occur in two steps, one of which is slow and the other fast: A+BCA+CD+Efastslow What is the rate law predicted by this mechanism? a) Rate =k[A][B] b) Rate =k[A][C] c) Rate =k[A]2[B] d) Rate =k[A][C]2 e) Rate =k[A]2[B][C] Answer Explanation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


