Question: Please explain the solution to this question about a process heater that uses natural gas with a lower heating value. If available, please provide where
Please explain the solution to this question about a process heater that uses natural gas with a lower heating value. If available, please provide where equation used is available in the NCEES reference manual. How should I know Equlibrium favors the formation of NO at higher temperatures.
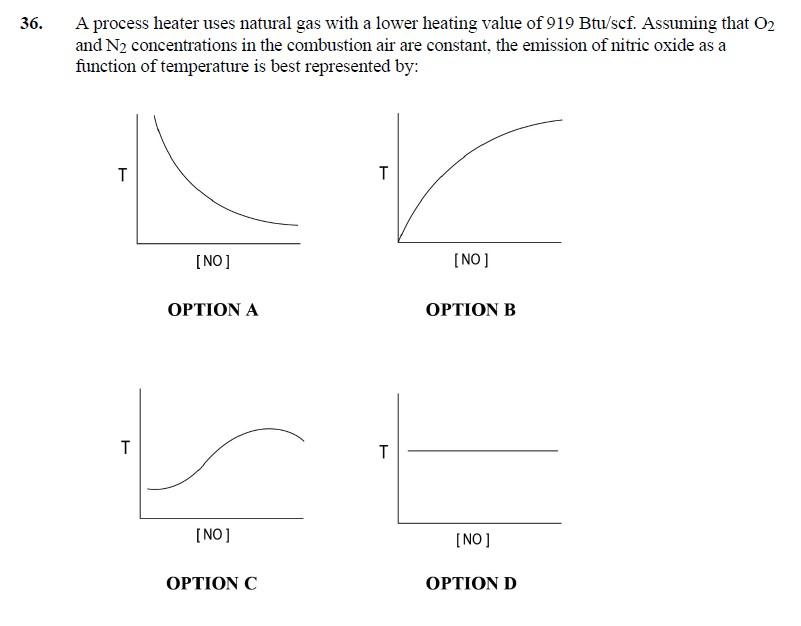
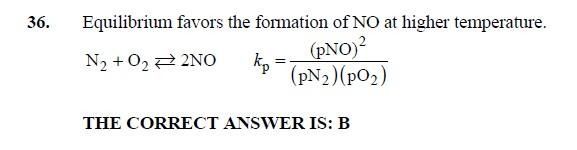
6. A process heater uses natural gas with a lower heating value of 919Btu/scf. Assuming that O2 and N2 concentrations in the combustion air are constant, the emission of nitric oxide as a 36. Equilibrium favors the formation of NO at higher temperature. N2+O22NOkp=(pN2)(pO2)(pNO)2 THE CORRECT ANSWER IS: B
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


