Question: Please explain this answer and name this molecule The UV spectrum of 1-phenylprop-2-en-1-ol shows an intense absorption at 220 nmnm and a weaker absorption at
Please explain this answer and name this molecule
The UV spectrum of 1-phenylprop-2-en-1-ol shows an intense absorption at 220 nmnm and a weaker absorption at 258 nmnm. When this compound is treated with dilute sulfuric acid, it rearranges to an isomer with an intense absorption at 250 nmnm and a weaker absorption at 290 nmnm. Suggest a structure for the isomeric product. Propose a mechanism for the formation of the structure!! (mechanism... electron flow esp)
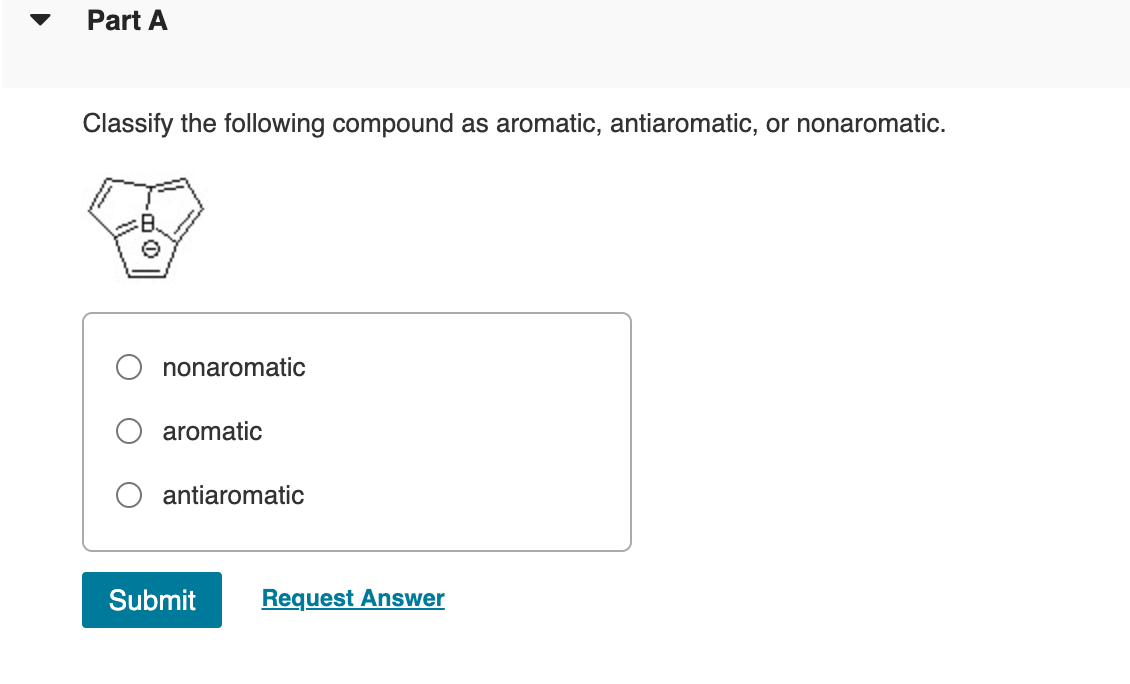
Part A Classify the following compound as aromatic, antiaromatic, or nonaromatic. nonaromatic aromatic antiaromatic Submit Request
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


