Question: please explain Tonsider these two entries from a fictional table of standard reduction potentials. X2+(aq)+2eX(s)Y2+(aq)+2eY(s)E=1.86VE=0.16V Which pair of species will react under standard conditions at
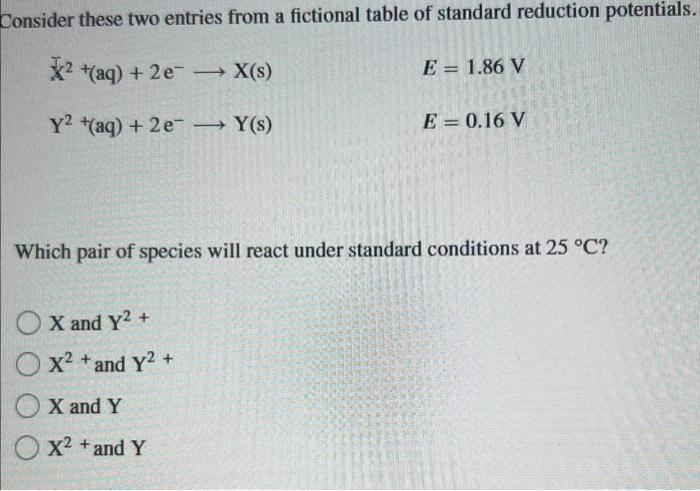
Tonsider these two entries from a fictional table of standard reduction potentials. X2+(aq)+2eX(s)Y2+(aq)+2eY(s)E=1.86VE=0.16V Which pair of species will react under standard conditions at 25C ? X and Y2+ X2+ and Y2+ X and Y X2+ and Y
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


