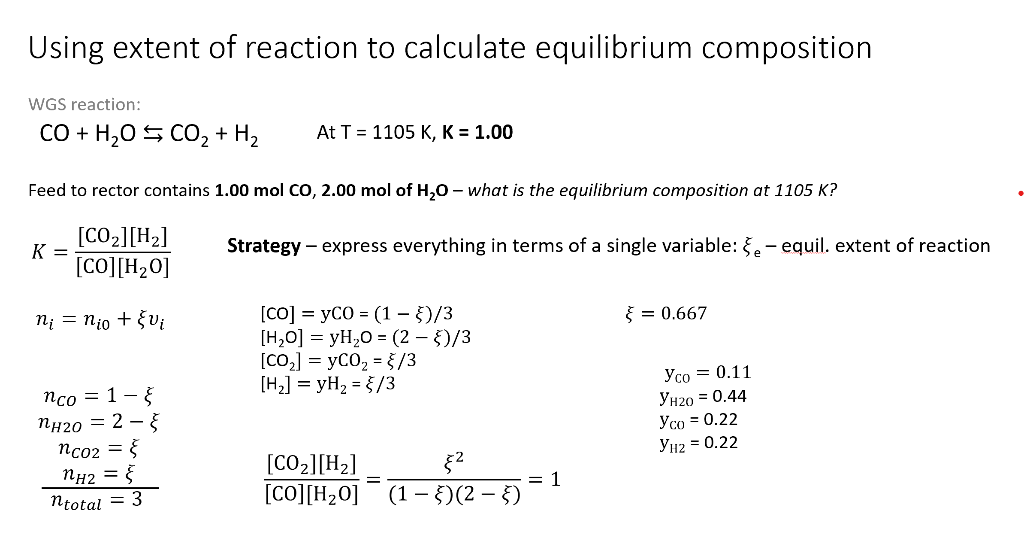
Question: Please explain whats going on here i have no idea Using extent of reaction to calculate equilibrium composition WGS reaction: CO+H2OCO2+H2AtT=1105K,K=1.00 Feed to rector contains
 Please explain whats going on here i have no idea
Please explain whats going on here i have no idea
Using extent of reaction to calculate equilibrium composition WGS reaction: CO+H2OCO2+H2AtT=1105K,K=1.00 Feed to rector contains 1.00molCO,2.00mol of H2O - what is the equilibrium composition at 1105K ? K=[CO][H2O][CO2][H2]Strategy-expresseverythingintermsofasinglevariable:e-equil.extentofreactiorni=ni0+vi[CO]=yCO=(1)/3=0.667[H2O]=yH2O=(2)/3[CO2]=yCO2=/3nCO=1[H2]=yH2=/3yCO=0.11yH20=0.44nH2O=2yC0=0.22nCO2=ntotal=3nH2=[CO][H2O][CO2][H2]=(1)(2)2=1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


