Question: Please explain why the answer for this question is 81.6 Garrett gets a job at an aerospace company after graduation from Penn State. The company
Please explain why the answer for this question is 81.6
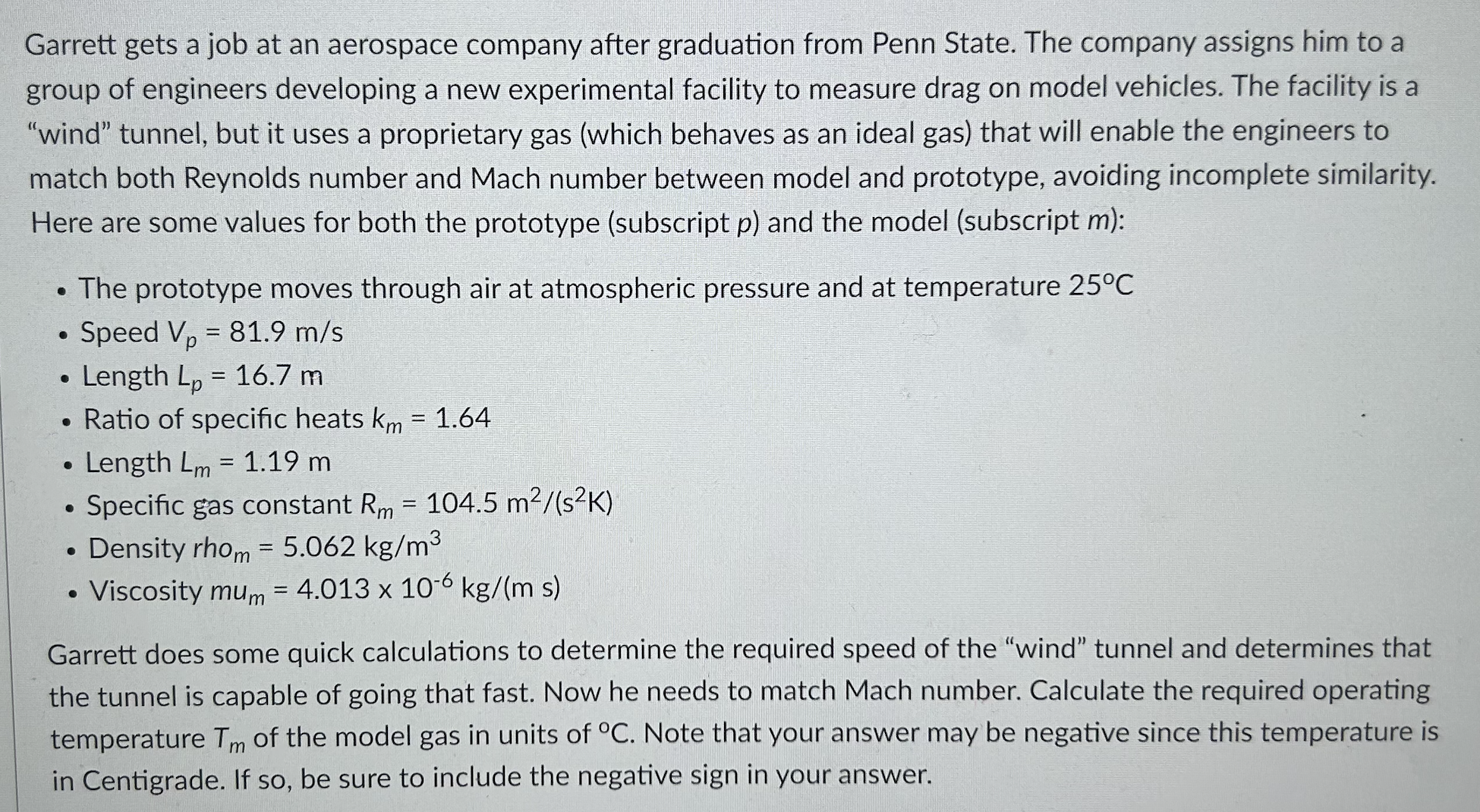
Garrett gets a job at an aerospace company after graduation from Penn State. The company assigns him to a group of engineers developing a new experimental facility to measure drag on model vehicles. The facility is a "wind" tunnel, but it uses a proprietary gas (which behaves as an ideal gas) that will enable the engineers to match both Reynolds number and Mach number between model and prototype, avoiding incomplete similarity. Here are some values for both the prototype (subscript p) and the model (subscript m): . The prototype moves through air at atmospheric pressure and at temperature 25 C . Speed Vp = 81.9 m/s . Length Lp = 16.7 m . Ratio of specific heats km = 1.64 . Length Lm = 1.19 m . Specific gas constant Rm = 104.5 m2/(s2K) . Density rhom = 5.062 kg/m3 . Viscosity mum = 4.013 x 10-6 kg/(m s) Garrett does some quick calculations to determine the required speed of the "wind" tunnel and determines that the tunnel is capable of going that fast. Now he needs to match Mach number. Calculate the required operating temperature Tm of the model gas in units of C. Note that your answer may be negative since this temperature is in Centigrade. If so, be sure to include the negative sign in your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


