Question: Please fill in the missing values in the table above. The show working where possible as I'm trying to understand where I went wrong thank
Please fill in the missing values in the table above. The show working where possible as I'm trying to understand where I went wrong thank you.
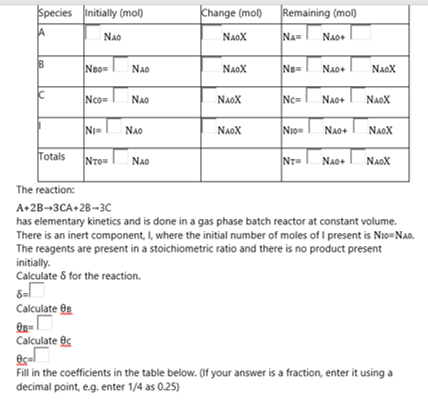
Change (mol) Remaining (mol) Species initially (mol) A NAD NAOX NA NAO B B NB NAO NAOX New NAO NAOX C Nos NAD NAOX Nc= NAO+ NAOX NI NAO NAOX NIO NAO+ NAOX Totals NAD NTE Nao+ NAOX The reaction: A+2B-3CA+2B-3C has elementary kinetics and is done in a gas phase batch reactor at constant volume. There is an inert component, I, where the initial number of moles of I present is NowNo. The reagents are present in a stoichiometric ratio and there is no product present initially Calculate for the reaction. Calculate O 08- Calculatec es Fill in the coefficients in the table below. If your answer is a fraction, enter it using a decimal point, e.g. enter 1/4 as 0.25)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


