Question: please fill in the table!!! and write the equations and process on how you did it! Write your compound in Table 2. Calculate how to
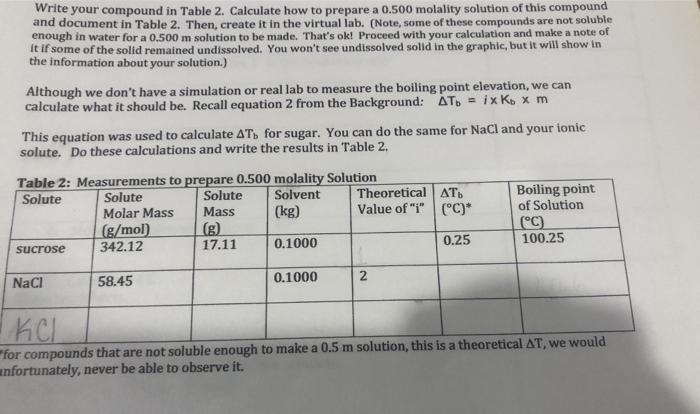
Write your compound in Table 2. Calculate how to prepare a 0.500 molality solution of this compound and document in Table 2. Then, create it in the virtual lab. (Note, some of these compounds are not soluble enough in water for a 0.500 m solution to be made. That's ok! Proceed with your calculation and make a note of it if some of the solid remained undissolved. You won't see undissolved solid in the graphic, but it will show in the information about your solution.) Although we don't have a simulation or real lab to measure the boiling point elevation, we can calculate what it should be. Recall equation 2 from the Background: AT = ix Ko x m This equation was used to calculate AT, for sugar. You can do the same for NaCl and your ionic solute. Do these calculations and write the results in Table 2. Table 2: Measurements to prepare 0.500 molality Solution Solute Solute Solute Solvent Theoretical AT. Boiling point Molar Mass Mass (kg) Value of "i" (C)* of Solution (g/mol) (C) sucrose 342.12 17.11 0.1000 0.25 100.25 NaCl 58.45 0.1000 2 for compounds that are not soluble enough to make a 0.5 m solution, this is a theoretical AT, we would unfortunately, never be able to observe it
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


